Abstract
Neuroplastin-65 and -55 (previously known as gp65 and gp55) are glycoproteins of the Ig superfamily that are enriched in rat forebrain synaptic membrane preparations. Whereas the two-Ig domain isoform neuroplastin-55 is expressed in many tissues, the three-Ig domain isoform neuroplastin-65 is brain-specific and enriched in postsynaptic density (PSD) protein preparations. Here, we have assessed the function of neuroplastin in long-term synaptic plasticity. Immunocytochemical studies with neuroplastin-65-specific antibodies differentially stain distinct synaptic neuropil regions of the rat hippocampus with most prominent immunoreactivity in the CA1 region and the proximal molecular layer of the dentate gyrus. Kainate-induced seizures cause a significant enhancement of neuroplastin-65 association with PSDs. Similarly, long-term potentiation (LTP) of CA1 synapses in hippocampal slices enhanced the association of neuroplastin-65 with a detergent-insoluble PSD-enriched protein fraction. Several antibodies against the neuroplastins, including one specific for neuroplastin-65, inhibited the maintenance of LTP. A similar effect was observed when recombinant fusion protein containing the three extracellular Ig domains of neuroplastin-65 was applied to hippocampal slices before LTP induction. Microsphere binding experiments using neuroplastin-Fc chimeric proteins show that constructs containing Ig1–3 or Ig1 domains, but not Ig2–3 domains mediate homophilic adhesion. These data suggest that neuroplastin plays an essential role in implementing long-term changes in synaptic activity, possibly by means of a homophilic adhesion mechanism.
Cell adhesion molecules (CAMs) are crucially involved in the assembly and restructuring of synapses during development and synaptic plasticity. Members of various CAM families are localized in synaptic junctions. These include: (i) members of the Ig superfamily, e.g., NCAM (neural CAM)-180 (1) and, in Drosophila, Fasciclin 2 (2); (ii) N- and E-cadherins, (3, 4) and novel cadherin-related proteins (5); (iii) integrins (6, 7); and (iv) the β-neurexin/neuroligin system (8, 9). Typically, synaptic CAMs span the synaptic cleft by homophilic or heterophilic interaction and are anchored to distinct cytoskeletal elements on either side of the synapse (10–14). They are thus well placed to mediate synapse formation and stabilization during development, and to participate in activity-induced synaptic plasticity, resulting in restructuring of synapses. Evidence for the latter is provided by the observation that the disturbance of synaptic cell adhesion, e.g., in mutant animals or by the application of antibodies or competitive peptides, in many cases interferes with long-term changes of synaptic plasticity. This has been reported for Ig superfamily CAMs NCAM180 and L1 (15, 16), telencephalin (17), Fasciclin 2 (18), and Aplysia apCAM (19), as well as for cadherins (20) and integrins (21, 22).
The neuroplastins are major glycoprotein components of synaptic membrane (SM) preparations belonging to the Ig superfamily (23). They originally were identified by using the mAb SMgp65, which was generated against SM glycoprotein preparations (24, 25). They occur as two isoforms, neuroplastin-65 (np65) and neuroplastin-55 (np55)—previously designated gp65 and gp55—and contain two and three extracellular Ig domains, respectively. The 65-kDa, but not the 55-kDa, isoform is enriched in rat forebrain postsynaptic density (PSD) preparations. Whereas np55 is expressed in most rat tissues, np65 is brain-specific (23, 26). Transcripts of both isoforms are widely distributed in rat brain. However, whereas np55 is present in all brain regions, np65 is concentrated in subpopulations of predominantly forebrain neurons and is enriched in neuropil regions (23, 24).
Both proteins are highly glycosylated and occur as multiple glycoforms (23–26). Interestingly, a fraction of np65 carries fucose α (1–2)-linked to galactose in its carbohydrate moiety (27). Fucosylated glycostructures have been implicated in long-term memory formation in different species (28–30) as well as in maintenance of hippocampal long-term potentiation (LTP) (31). Here, we assessed the role of neuroplastin in processes of synaptic plasticity. We provide strong evidence that the association of np65 with the PSD fraction is regulated by synaptic activity and that neuroplastin is involved in plasticity-dependent synaptic restructuring.
Materials and Methods
Recombinant Proteins and Antibodies.
A segment of the cDNA of np55 encoding amino acid residues −2 to 192 (23) was amplified by PCR and cloned into the bacterial expression vector pQE30 (Qiagen, Chatsworth, CA). Similarly, a np65 cDNA segment encoding amino acid residues 3–308 was introduced into pQE30. From these constructs, recombinant proteins of either 26 kDa (Ig domains 2–3) or 40 kDa (Ig domains 1–3) containing six N-terminal histidine residues were expressed (Fig. 1A). The first Ig domain of np65 (amino acids 3–118) was cloned into the pGEXλ1T vector (Amersham Pharmacia) to produce a 42-kDa glutathione S-transferase fusion protein. Purified fusion proteins were used to produce the polyclonal rabbit antisera AS Ig1 against the first np65-specific Ig domain, AS Ig2–3 against Ig domains 2–3, and AS Ig1–3 against Ig domains 1–3.
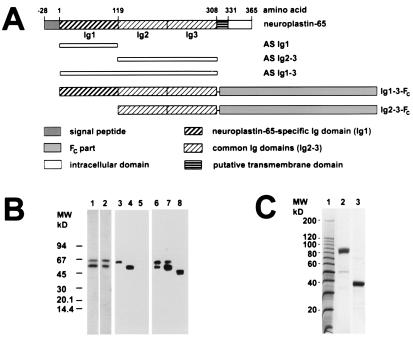
Figure 1.
Characterization of neuroplastin antibodies and fusion proteins. (A) Map of np65 (np55 lacks the first Ig domain). The recombinant fragments to produce rabbit antisera AS Ig1, AS Ig2–3, and AS Ig1–3 are indicated below the map. The epitope for mAb SMgp65 is located within the Ig2–3 region. The Ig1–3-Fc and Ig2–3-Fc fusion proteins produced in 293 cells are also shown. (B) Specificity of antibodies. Western blots of Triton X-100-solubilized membrane proteins from rat brain (lanes 1–3 and 6), np65-transfected 293 cells (lanes 4 and 7), and np55-transfected 293 cells (lanes 5 and 8) were probed with the following antibodies: AS Ig2–3 (lane 1), AS Ig1–3 (lane 2), AS Ig1 (lanes 3–5), and mAb SMgp65 (lanes 6–8). Note, differential glycosylation of neuroplastins expressed by 293 cells results in size differences from neuroplastins of brain membrane extracts (23, 26). mAb SMgp65 recognizes two glycoforms of np65 in transfected 293 cells (lane 7, and ref. 23). (C) Purification of the Fc-fusion proteins. Affinity-purified fusion protein Ig1–3-Fc (lane 2) and human Fc alone (lane 3) were separated by SDS/PAGE and stained with Coomassie brilliant blue. Molecular weight markers are shown in lane 1.
mAb SMgp65 was produced against ConA-binding SM glycoproteins from rat brain (24). IgG fractions from antisera were affinity-purified on GammaBind Plus Sepharose (Amersham Pharmacia) and buffer-exchanged into artificial cerebrospinal fluid (ACSF; ref. 32) on FPLC fast-desalting columns (Amersham Pharmacia).
Eukaryotic recombinant proteins containing the Ig domains of np65 (amino acids 3–307) or np55 (amino acids 3- 191) fused to the Fc region of human IgG were produced in 293 cells. The ORF were amplified by PCR from neuroplastin cDNAs as above and cloned in-frame behind the BM40 signal peptide (33) into the eukaryotic expression vector pIgPlus (provided by P. Doherty, King's College, London). To express the Fc region of human IgG alone, the BM40 signal sequence was fused directly to the Fc sequence of pIgPlus. Cell lines stably expressing and secreting the fusion proteins were generated as described (23). Fusion proteins were purified from culture supernatants by affinity chromatography using GammaBind Plus Sepharose. For LTP experiments, the buffer was exchanged as described for the antibodies.
Immunohistochemistry.
Immunohistochemical experiments were performed as described (34). Np65 was detected in 7-μm frontal and sagittal sections by using AS Ig1 (1:300) as primary antibody (22 h), followed by incubation with porcine anti-rabbit IgG (Dako) diluted 1:50 (30 min) and rabbit peroxidase-antiperoxidase complex (Dako) diluted 1:100 (30 min). Immunoreactivity then was visualized by using 3,3-diaminobenzidine (0.05%)/H2O2 (0.001%) (Sigma).
Kainate Treatment of Rats.
Twelve-week-old male Wistar rats were injected i.p. with kainate (13 mg/kg body weight) or saline and killed 6 h later (35). Seizure development was assessed to distinguish control, kainate-treated nonseizure, and the kainate-treated seizure groups of rats. For preparation of synaptic proteins, cortices and hippocampi of three animals were pooled for one sample. All animal experiments were performed in accordance with the guidelines and regulations defined by the German Animal Welfare Act. Permission was obtained from the Regierungspraesidium Dessau.
Isolation and Analysis of Subcellular Protein Fractions.
Synaptic proteins (PSD fraction) were prepared essentially as described by Carlin et al. (36) with modifications as detailed in ref. 37. For quantification of neuroplastin immunoreactivity in a PSD-enriched protein fraction after induction of LTP, hippocampal slices were stimulated as described (38). Two hours after tetanization, 10 control or stimulated slices were pooled and homogenized in 200 μl of PSD-extraction buffer (37) and kept for 1 h at 4°C to solubilize noncytoskeletal, non-PSD protein. Subsequently, samples were spun for 1 h at 100,000 × g, and the pellets were rehomogenized in extraction buffer and washed by centrifugation at 100,000 × g. The resulting pellet is enriched in PSD proteins.
Electrophysiology.
LTP experiments were performed on transverse slices (400 μm) of the right hippocampus from 8-wk-old male Wistar rats, strain Shoe, as described (32). Antibodies, recombinant protein, or appropriate control solutions were applied onto the CA1 stratum radiatum by using a microinfusion pump (delivery rate of ≈0.75 μl/min) for 80 min. After 60 min of antibody infusion, LTP was induced by using three 100-Hz stimulus trains, each containing 50 pulses at double-pulse width with a 2-min interval between each train. Field excitatory postsynaptic potentials (fEPSP) were recorded in 5- to 10-min intervals for at least 3 h. LTP magnitude was calculated as percent change of fEPSP as compared with averaged baseline responses measured during 30 min before drug application (mean ± SEM). Statistical evaluation of the data was performed with a two-tailed Mann–Whitney U test.
Microsphere Binding Assays.
Assays were carried out essentially as described (39). Briefly, anti-human Fc (Sigma) was passively adsorbed onto 0.6-μm diameter red covaspheres (Duke Scientific, Palo Alta, CA) or 1-μm diameter yellow-green Fluoresbrite microspheres (Polysciences) in PBS for 1 h. Microspheres were washed for 2 min in PBS (three times) and pelleted. Subsequently, uncoupled sites were blocked by incubation in 5% FCS (GIBCO/BRL) for 1 h. After washing as above, beads were incubated with appropriate Fc construct for 1 h, washed, and resuspended in PBS (50 μl). All incubations were at room temperature. Fc-coated microspheres were further diluted (10 μl into 50 μl PBS), sonicated on ice for 10 min, followed by incubation for 60 min at room temperature to allow aggregation. Samples (6 μl, three samples per time point) were taken at 15, 30, 45, and 60 min and diluted in 1 ml of PBS. Three aliquots (100 μl per sample) were transferred to 96-well plates, and the plates were centrifuged at 2,750 rpm for 10 min. Aggregation was monitored with a Leica fluorescent microscope. Images were captured and analyzed by using photolite and image proplus software (Media Cybernetics, Silver Spring, MD). For all experiments, aggregation was determined as percent decrease in nonaggregated beads relative to the number of nonaggregated beads coated with Fc alone at time 0.
Results
Characterization of Neuroplastin Antibodies.
Various antibodies directed against different extracellular domains of neuroplastin were used to study the distribution and the function of these molecules in the rat hippocampus. Antibodies include rabbit antisera raised against bacterial recombinant protein corresponding to the np65-specific, the two common, or all three Ig domains (AS Ig1, AS Ig1–3, 2–3) (Fig. 1A), and mAb SMgp65, which recognizes both isoforms on immunoblots (24). In rat brain membrane preparations, AS Ig2–3, AS Ig1–3, and mAb SMgp65 recognize two bands of 55 and 65 kDa (Fig. 1B, lanes 1, 2, and 6), whereas AS Ig1 detects a single band of 65 kDa (Fig. 1B, lane 3). The specificity of AS Ig1 for np65 was further confirmed by showing that this antiserum detects an immunoreactive band only on Western blots from np65-transfected but not from np55-transfected 293 cells (Fig. 1B, lanes 4 and 5). Consistent with previous data (23), mAb SMgp65 reacts with polypeptides expressed by both cell lines (Fig. 1B, lanes 7 and 8).
Localization of np65 in the Rat Forebrain.
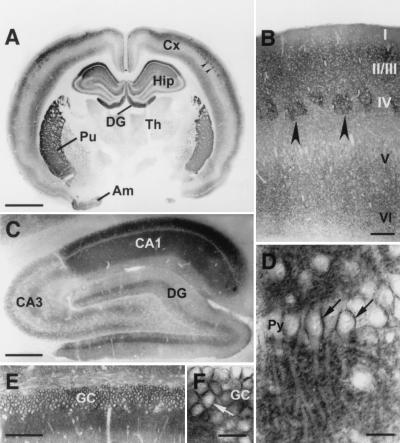
The np65-specific AS Ig1 was used to establish the distribution of this isoform in rat forebrain. An overall view of a frontal section shows that np65 is prominently expressed in the cerebral cortex, the hippocampus, the amygdala, and the striatum. Most intense staining within the latter structure is found in the putamen (Fig. 2A). In the cortex, neuropil regions of layers II, III, and Vb/VI are prominently stained; other layers are moderately labeled (Fig. 2 A and B). In the somatosensory cortex, barrel fields in layer IV exhibit particularly strong staining (arrowheads, Fig. 2 A and B). Moderate staining also is observed in the medial and lateral thalamic nuclei. The overall distribution of the 65-kDa isoform of neuroplastin is consistent with the distribution of np65-specific transcripts (23).
Figure 2.
Distribution of np65 in the forebrain detected with np65-specific antibodies (AS Ig1). (A) Frontal section of rat forebrain. Intense immunoreactivity is found in the cerebral cortex (Cx), the hippocampal formation (Hip), including dentate gyrus (DG), the putamen (Pu), and the amygdala (Am). Th, thalamus. (B) Frontal section of somatosensory cortex; layering is indicated. Arrowheads in A and B mark strongly stained barrel fields. (C) Sagittal section of the hippocampus. CA1, CA3, Ammon's horn regions 1 and 3. (D–F) Enlargements of the CA1 pyramidal cell layer (Py) and the granule cell layer (GC) of the DG indicate cell surface staining of these neurons (arrows). (Size bars: 2 mm in A; 200 μm in B; 500 μm in C; 25 μm in D and F; 100 μm in E.)
In the hippocampus, significant regional differences in np65-specific immunoreactivity were observed. The most prominent staining was found in the neuropil layers of the CA1 region and the dentate gyrus (Fig. 2 A and C–F). In CA1, the stratum oriens and the stratum radiatum display much stronger immunoreactivity than the stratum lacunosum moleculare (Fig. 2C). A high-power view (Fig. 2D) shows that the neuropil staining is punctate and that somata of CA1 pyramidal neurons are surrounded by immunoreactivity. In contrast, the CA3 region shows only moderate staining that is laminar in appearance (Fig. 2C). Note the sharp boundary between the intensely immunoreactive CA1 and moderately immunoreactive CA3 regions. In the dentate gyrus, strong np65 immunoreactivity is found in the inner molecular layer, whereas the middle and outer molecular layers are less intensely stained (Fig. 2 C and E). The staining of the inner molecular layer is likely to come from projections of mossy cells of the hilar polymorphic layer (40). Granule cells, like pyramidal cells, display significant cell surface staining (Fig. 2F).
Synaptic Activity Regulates Association of np65 with the Synaptic Protein (PSD) Fraction.
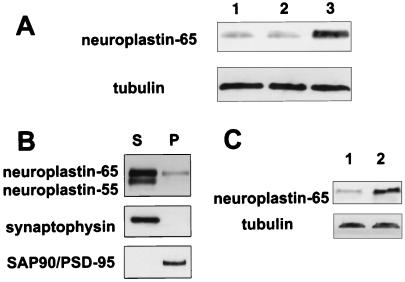
As np65 is a putative PSD-associated CAM, we investigated whether the association of this isoform with the PSD is regulated by synaptic activity. Treatment of animals with kainate induces a widespread increase in synaptic activity, which results in generalized tonic-clonic seizures (41, 42). As is well-established, kainate treatment induces seizures (stages 5–6) in some, but not all, animals. The neuroplastin content of the PSD fractions prepared from seizure, nonseizure, and control groups of animals 6 h after kainate treatment was determined by immunoblot analysis. The relative amount of np65 is significantly increased in the PSD fraction of seizure animals 4- to 5-fold, as compared with nonseizure and control rats (Fig. 3A). The relative amount of tubulin in the PSD fractions remains essentially unchanged. Light microscopy immunohistochemistry revealed no major redistribution of np65 distribution after kainate treatment (not shown).
Figure 3.
np65 content of synaptic protein fraction is regulated by synaptic activity. (A) Enhanced np65 immunoreactivity in the rat brain PSD fraction after kainate-induced seizure. Immunoblots of PSD proteins from untreated animals (lane 1), kainate-treated, nonseizure (lane 2), and kainate-treated seizure animals (lane 3) were developed with mAb SMgp65 or anti-tubulin antibodies. (B) Preparation of PSD-enriched fraction from hippocampal slices. Ten hippocampal slices were pooled and extracted with PSD-extraction buffer. Western blots of soluble fraction (S) and insoluble pellet fraction (P) were probed with mAb SMgp65, and antibodies against synaptophysin and SAP90/PSD-95. Note, a fraction of np65, but not np55, is detected in the PSD-enriched fraction. (C) LTP enhances np65 immunoreactivity in PSD-enriched fraction. Western blots of PSD-enriched protein fractions from untreated and tetanized (2 h after tetanization) hippocampal slices were probed with SMgp65 and anti-tubulin antibody (control, lane 1; LTP, lane 2). Tetanization results in a 190 ± 39% increase (n = 6; Wilcoxon test, P < 0,03) of np65 in the PSD-enriched fraction.
In another series of experiments, we tested whether the association of np65 with synaptic protein fractions is regulated by LTP in acutely isolated hippocampal slices. Because of the limited amount of tissue, it was not practical to prepare conventional PSD fractions from individual slices. Therefore, a detergent-insoluble PSD-enriched fraction was isolated. PSD enrichment in this fraction was confirmed by the PSD marker protein SAP90/PSD95 and by the absence of the synaptic vesicle protein synaptophysin in the pellet (Fig. 4B). As expected (24), a fraction of np65, but not np55, is found in the PSD-enriched fraction (Fig. 3B).
Figure 4.
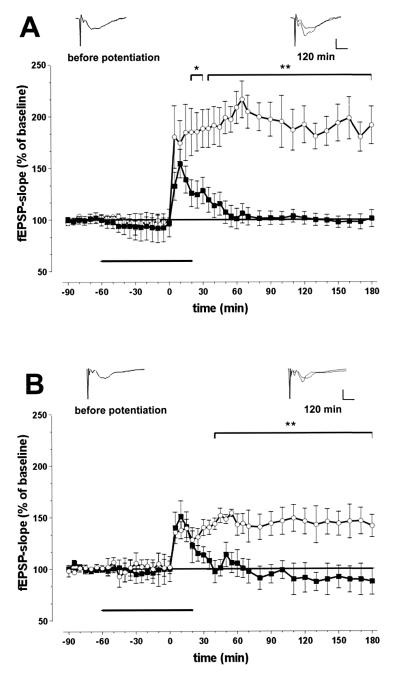
Antibodies against np65 (A) and recombinant neuroplastin extracellular domains (B) prevent maintenance of LTP at CA1 synapses in hippocampal slices. (A) Ensemble average of fEPSPs of all experiments (n = 7) with slices superfused with antibody AS Ig1 (■) or with ACFS containing rabbit Igs (n = 8; ○). (B) Ensemble average of fEPSPs of all experiments (n = 7) with slices perfused with recombinant fusion protein Ig1–3-Fc (■) or with the Fc fragment alone (n = 6; ○). Superimposed representative samples of fEPSPs taken 10 min before and 120 min after tetanus are inserted in A and B. (Scale bar: 2 mV, 2 ms.)
Immunoblots of PSD-enriched fractions prepared from untreated and tetanized (2 h after tetanization) hippocampal slices were probed with neuroplastin antibodies. Tetanization results in a ≈2-fold increase of np65 in the PSD-enriched fraction (Fig. 3C). After 6 h, still an ≈1.3-fold increase is observed (not shown). No LTP-induced change was observed for tubulin (Fig. 3C).
Neuroplastin Antibodies and Recombinant Protein Block the Maintenance of LTP.
Four different antibodies against various extracellular domains of neuroplastin (Fig. 1) were used to examine whether the protein is directly involved in LTP at CA1 synapses. Basal fEPSP responses recorded for 90 min were not affected by this treatment. Stimulation of the Schaffer collaterals with three subsequent 100-Hz-stimulus trains induced stable LTP for at least 3 h under control conditions, i.e., in the presence of ACSF or ACSF-containing control Igs (Fig. 4A). Application of neuroplastin antibodies did not prevent induction of LTP, although fEPSPs in some cases appear to be somewhat reduced 5 min after stimulation. All four neuroplastin antibodies inhibited maintenance of LTP. Data are illustrated for the np65-specific antibody AS Ig1 (Fig. 4A) and summarized in Table 1. Ten minutes after tetanization, fEPSPs in experimental slices were not significantly different from controls. Thereafter, the potentiated fEPSPs declined. They became significantly lower than those in stably potentiated controls 30 min after tetaniziation and reached baseline within 1 h. In addition, the recombinant neuroplastin-Ig1–3-Fc caused rapid decay of potentiation between 10 and 30 min after tetanization and prevented maintenance of LTP (Fig. 4B and Table 1). Fc alone did not have this effect.
Table 1.
Antibodies against different Ig domains of neuroplastin and the recombinant fusion protein Ig1-3-Fc prevent maintenance of hippocampal CA1 LTP
| % Increase in fEPSP (mean ± SEM)
|
|||||
|---|---|---|---|---|---|
| 5 min | 10 min | 30 min | 60 min | 120 min | |
| Rabbit IgG fraction (n = 8) | 180.2 ± 30.2 | 172.2 ± 21.9 | 188.2 ± 18.2 | 208.5 ± 15.5 | 180.0 ± 14.1 |
| AS Ig1 (n = 7) | 121.9 ± 24.8 | 153.6 ± 24.6 | 124.5 ± 20.6** | 102.6 ± 18.4** | 101.8 ± 9.5** |
| AS Ig1–3 (n = 8) | 159.0 ± 18.5 | 166.3 ± 20.1 | 138.8 ± 14.8** | 124.6 ± 11.3** | 100.9 ± 4.1** |
| AS Ig2–3 (n = 8) | 132.5 ± 13.5 | 154.4 ± 13.8 | 128.2 ± 12.8* | 100.9 ± 10.0** | 104.4 ± 6.1** |
| Control IgG fraction (n = 7) | 163.3 ± 9.3 | 162.7 ± 5.4 | 181.8 ± 16.8 | 187.0 ± 19.0 | 175.2 ± 39.0 |
| SMgp65 (n = 7) | 139.7 ± 17.6 | 160.2 ± 31.9 | 146.8 ± 28.7* | 139.2 ± 21.6* | 108.1 ± 8.4** |
| hum IgG-Fc (n = 6) | 135.3 ± 5.3 | 137.4 ± 8.2 | 140.0 ± 7.7 | 142.8 ± 10.4 | 145.9 ± 29.4 |
| Ig1–3Fc (n = 7) | 139.8 ± 15.3 | 150.9 ± 15.0 | 113.6 ± 7.8† | 105.4 ± 13.0†† | 91.3 ± 14.3†† |
Antibodies, recombinant protein, or appropriate control solutions were applied from 60 min before to 20 min after high-frequency stimulation. Data represent percent change of the fEPSP as compared to averaged baseline responses measured during 30 min before application (mean ± SEM). *, P < 0.05; **, P < 0.02 as compared to ACSF containing IgG fraction from rabbit normal serum; *, P < 0.05; **, P < 0.02 as compared to IgG fraction from hybridoma culture medium; †, P < 0.05; ††, P < 0.02 as compared to human IgG-Fc.
Recombinant np65-Fc Fusion Proteins Display Homophilic Adhesion.
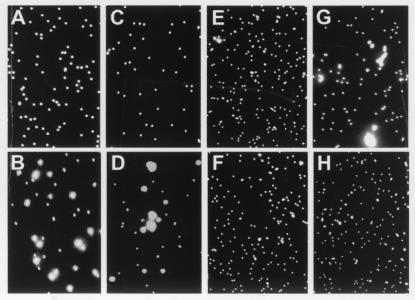
Many Ig superfamily adhesion molecules, including NCAM and L1, mediate homophilic binding (43, 44). Microsphere binding assays were carried out to determine whether this is the case for np65 and np55. Fluorescent microspheres coated with Fc chimeric proteins containing all three (Ig1–3-Fc) or the two common Ig (Ig2–3-Fc) domains were used to test this possibility. The results show that minimal aggregation (1.25 ± 0.31%; n = 9) was observed for beads coated with the Fc portion of human Ig alone (Fig. 5A). As expected reproducible aggregation was observed for microspheres coated with chimeric NCAM-human Fc protein (Fig. 5B). Aggregation also was observed for beads coated with Ig1–3-Fc, but not Ig2–3-Fc (Fig. 5 D and C). The percent aggregation observed with the NCAM and Ig1–3-Fc constructs were 43.61 ± 0.55% (n = 9) and 37.28 ± 0.31% (n = 9), respectively. The specificity of the aggregation observed with Ig1–3-Fc was tested by carrying out the binding assay in the presence of SMgp65 antibody or by heat-denaturing the construct in the presence of 2-mercaptoethanol before carrying out the assay (Fig. 5 E and F). In both cases, aggregation was blocked and did not differ significantly from that observed for the Fc-coated beads. These data suggest that the Ig-1 domain is involved in the homophilic binding of np65. Indeed, aggregation (34.58 ± 0.10%, n = 9) of beads coated with Ig1-Fc is observed (Fig. 5G). This aggregation is significantly reduced (23.30 ± 0.63%, P > 0.001, n = 9) by inclusion of the np65-specific antiserum AS Ig1 in the assay medium.
Figure 5.
Homophilic aggregation assay. Microspheres were coated with: (A) 5 μg Fc alone; (B) 4 μg NCAM-Fc; (C) 5 μg Ig2–3-Fc; (D) 5 μg Ig1–3-Fc; (E) Ig1–3-Fc in the presence of mAb SMgp65 (10 μl hybridoma supernatant per 50-μl assay volume); (F) Ig1–3-Fc denatured by boiling for 5 min in the presence of 1% (vol/vol) β-mercaptoethanol. (G) Fluoresbrite microspheres coated with 5 μg Ig1-Fc; (H) Ig1-Fc (5 μg)-coated microspheres in the presence of AS Ig1 (5 μl). In all panels, aggregation is shown after 60 min assay time. Note that NCAM (B), Ig1–3-Fc (D), and Ig1-Fc (G), but not Ig2–3-Fc (C) mediate aggregation.
Discussion
Neuroplastin has long been known as a synaptic glycoprotein (24); however, its functions have been unknown. Here, we provide strong evidence that np65 is involved in plasticity at hippocampal synapses. First, np65 is highly and differentially expressed in the hippocampus with most prominent appearance in the synaptic neuropil region of CA1. Second, a significant increase in np65 immunoreactivity is detected in PSD-enriched and PSD fractions after LTP induced in hippocampal slices or after seizures induced by kainate, respectively. Recent studies have shown that changes of synaptic efficacy in the brain, e.g., as induced by ischemia, can induce changes in synaptic structure and in the protein composition of PSDs (45, 46). Kainate is known to strongly affect synaptic activity in limbic structures, including pyramidal neurons of the hippocampus (41, 42), and synaptic restructuring was described to take place after LTP (47–49). The observed enhanced association of np65 with synaptic structures may reflect an involvement of the molecule in these plasticity-induced restructuring processes. Third, the perturbation experiments with antibodies and recombinant polypeptide that almost completely suppress the maintenance of LTP at CA1 synapses provide the most direct evidence for the involvement of neuroplastin in synaptic plasticity. One of the applied antibodies exclusively recognizes np65, further supporting the view that this isoform is crucially involved in synaptic effects of neuroplastin. The most plausible explanation of antibody-mediated suppression of long-term changes of synaptic strength is that they interfere with existing or newly established protein–protein interactions mediated by the antigens. A similar competitive disruptive effect may be achieved by the use of peptides or recombinant protein domains, which mimic natural binding partners. This has been reported e.g., for integrins (21, 22) and cadherins (20), as well as for the Ig superfamily member telencephalin (17).
Currently, the mechanism by which neuroplastin affects synaptic plasticity is unknown. Our interaction studies in vitro provide clear evidence that np65, but not np55, mediates homophilic binding, and that the interaction is blocked by antineuroplastin antibody, which also blocks LTP. The 65-kDa isoform has an interaction potential similar to that of other Ig superfamily adhesion molecules, including NCAM and L1, which are known to mediate homophilic binding (43, 44). Both CA3 and CA1 pyramidal cells express np65 transcripts (23), suggesting that the protein could be present on both sides of synapses between Schaffer collaterals and CA1 pyramidal cell dendrites. Thus, a homophilic interaction across the synaptic cleft is conceivable, assuming a width of the cleft of 20–30 nm (50) and an extension into the cleft of np65 of about 13–16 nm, as suggested by molecular modeling of its three Ig domains (C. Reissner and E.D.G., unpublished data). However, in contrast to NCAM, where multiple Ig domains may be involved in homophilic interaction and bending of the protein has to be invoked to fit into the synaptic cleft (43), in np65, primarily the first isoform-specific Ig domain may mediate physical interaction. This is supported by the observation that Ig1-Fc mediates clustering of microspheres, and this response is significantly blocked by the np65-specific antiserum. Nonetheless, it must be stressed that the role of np55 in neuronal plasticity processes is not excluded by our experiments, as no tools specific for this isoform are available.
Interestingly, normal synaptic transmission appears essentially unaffected by the neuroplastin antibodies and Ig1–3-Fc fusion protein, and, also, short-term potentiation is possible in the presence of these agents. Thus, neuroplastin appears to be involved in long-term synaptic changes only. Similar observations have been made for other CAMs found to be involved in synaptic plasticity, including L1 and NCAM (15, 16, 51), telencepahlin (17), integrins (21, 22), cadherins (20), amyloid precursor protein (52, 53), and N-syndecan (54).
The time course of decay of synaptic potentiation produced by neuroplastin antibodies is reminiscent of that reported for protein kinase C (PKC) inhibitors (55, 56). Interestingly, the distribution of PKCγ immunoreactivity in the hippocampus (57) is remarkably similar to that of np65. Two Ig superfamily CAMs, NCAM and L1, have been implicated in PKC-dependent signaling processes in growth cones (58). Thus, it will be interesting to find out whether the observed effect of neuroplastin on LTP maintenance also involves PKC.
Recent evidence indicates that rapid changes in synaptic plasticity are accompanied by rapid ultrastructural modifications to or an altered number of synapses. Thus, the occurrence of dendritic protrusions and spine-like structures has been visualized in living hippocampal neurons in culture 30–60 min after LTP induction (59, 60). Furthermore, electron microscopic analyses of calcium precipitates in spines following thetaburst stimulation revealed that LTP promotes formation of new mature synapses contacting the same presynaptic terminal 1 h after tetanization (61). Such structural changes associated with long-term synaptic plasticity may depend on the action of a variety of very different CAMs and extracellular matrix components. Regulation mechanisms may include activity-driven regulation of CAM expression (62–64) as well as activity-regulated recruitment of NCAM180 (65) or np65 into the synapse to locally enhance physical interaction or to enlarge the contact area of the synaptic membranes and in turn cause strengthening of synapses.
Acknowledgments
We thank R. Grimm and W. Tischmeyer for providing kainate-treated animals and K. Richter, C. Otto, K. Zobel, K. Schulzeck, and R. Mummery for expert technical assistance. The pIgPlus vector was a gift from Prof. P. Doherty. This study was supported by the Deutsche Forschungsgemeinschaft (to M.K., S.S., and E.D.G.), the Volkswagen-Stiftung (to E.D.G. and U.W.), the Wellcome Trust (to P.W.B.), and a North Atlantic Treaty Organization travel grant (to P.W.B. and E.D.G.).
Abbreviations
- ACSF
artificial cerebrospinal fluid
- CAM
cell adhesion molecule
- NCAM
neural CAM
- fEPSP
field excitatory postsynaptic potential
- np65
neuroplastin-65
- np55
neuroplastin-55
- LTP
long-term potentiation
- PSD
postsynaptic density
- SM
synaptic membrane
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.080389297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.080389297
References
- 1.Persohn E, Pollerberg G E, Schachner M. J Comp Neurol. 1989;288:92–100. doi: 10.1002/cne.902880108. [DOI] [PubMed] [Google Scholar]
- 2.Schuster C M, Davis G W, Fetter R D, Goodman C S. Neuron. 1996;17:641–654. doi: 10.1016/s0896-6273(00)80197-x. [DOI] [PubMed] [Google Scholar]
- 3.Beesley P W, Mummery R, Tibaldi J. J Neurochem. 1995;64:2288–2294. doi: 10.1046/j.1471-4159.1995.64052288.x. [DOI] [PubMed] [Google Scholar]
- 4.Fannon A M, Colman D R. Neuron. 1996;17:423–434. doi: 10.1016/s0896-6273(00)80175-0. [DOI] [PubMed] [Google Scholar]
- 5.Kohmura N, Senzaki K, Hamada S, Kai N, Yasuda R, Watanabe M, Ishii H, Yasuda M, Mishina M, Yagi T. Neuron. 1998;20:1137–1151. doi: 10.1016/s0896-6273(00)80495-x. [DOI] [PubMed] [Google Scholar]
- 6.Einheber S, Schnapp L M, Salzer J L, Cappiello Z B, Milner T A. J Comp Neurol. 1996;370:105–134. doi: 10.1002/(SICI)1096-9861(19960617)370:1<105::AID-CNE10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 7.Nishimura S L, Boylen K P, Einheber S, Milner T A, Ramos D M, Pytela R. Brain Res. 1998;791:271–282. doi: 10.1016/s0006-8993(98)00118-8. [DOI] [PubMed] [Google Scholar]
- 8.Missler M, Sudhof T C. Trends Genet. 1998;14:20–26. doi: 10.1016/S0168-9525(97)01324-3. [DOI] [PubMed] [Google Scholar]
- 9.Song J Y, Ichtchenko K, Sudhof T C, Brose N. Proc Natl Acad Sci USA. 1999;96:1100–1105. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uchida N, Honjo Y, Johnson K R, Wheelock M J, Takeichi M. J Cell Biol. 1996;135:767–779. doi: 10.1083/jcb.135.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas U, Kim E, Kuhlendahl S, Koh Y H, Gundelfinger E D, Sheng M, Garner C C, Budnik V. Neuron. 1997;19:787–799. doi: 10.1016/s0896-6273(00)80961-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zito K, Fetter R D, Goodman C S, Isacoff E Y. Neuron. 1997;19:1007–1016. doi: 10.1016/s0896-6273(00)80393-1. [DOI] [PubMed] [Google Scholar]
- 13.Irie M, Hata Y, Takeuchi M, Ichtchenko K, Toyoda A, Hirao K, Takai Y, Rosahl T W, Sudhof T C. Science. 1997;277:1511–1515. doi: 10.1126/science.277.5331.1511. [DOI] [PubMed] [Google Scholar]
- 14.Butz S, Okamoto M, Sudhof T C. Cell. 1998;94:773–782. doi: 10.1016/s0092-8674(00)81736-5. [DOI] [PubMed] [Google Scholar]
- 15.Lüthi A, Laurent J P, Figurov A, Muller D, Schachner M. Nature (London) 1994;372:777–779. doi: 10.1038/372777a0. [DOI] [PubMed] [Google Scholar]
- 16.Cremer H, Chazal G, Carleton A, Goridis C, Vincent J D, Lledo P M. Proc Natl Acad Sci USA. 1998;95:13242–13247. doi: 10.1073/pnas.95.22.13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakurai E, Hashikawa T, Yoshihara Y, Kaneko S, Satoh M, Mori K. NeuroReport. 1998;9:881–886. doi: 10.1097/00001756-199803300-00022. [DOI] [PubMed] [Google Scholar]
- 18.Davis G W, Schuster C M, Goodman C S. Neuron. 1997;19:561–573. doi: 10.1016/s0896-6273(00)80372-4. [DOI] [PubMed] [Google Scholar]
- 19.Mayford M, Barzilai A, Keller F, Schacher S, Kandel E R. Science. 1992;256:638–644. doi: 10.1126/science.1585176. [DOI] [PubMed] [Google Scholar]
- 20.Tang L, Hung C P, Schuman E M. Neuron. 1998;20:1165–1175. doi: 10.1016/s0896-6273(00)80497-3. [DOI] [PubMed] [Google Scholar]
- 21.Staubli U, Vanderklish P, Lynch G. Behav Neural Biol. 1990;53:1–5. doi: 10.1016/0163-1047(90)90712-f. [DOI] [PubMed] [Google Scholar]
- 22.Staubli U, Chun D, Lynch G. J Neurosci. 1998;18:3460–3469. doi: 10.1523/JNEUROSCI.18-09-03460.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langnaese K, Beesley P W, Gundelfinger E D. J Biol Chem. 1997;272:821–827. doi: 10.1074/jbc.272.2.821. [DOI] [PubMed] [Google Scholar]
- 24.Hill I, Selkirk C P, Hawkes R B, Beesley P W. Brain Res. 1988;461:27–43. doi: 10.1016/0006-8993(88)90722-6. [DOI] [PubMed] [Google Scholar]
- 25.Willmott T, Skitsa I, Hill I, Mummery R, Beesley P W. J Neurochem. 1992;58:2037–2043. doi: 10.1111/j.1471-4159.1992.tb10944.x. [DOI] [PubMed] [Google Scholar]
- 26.Langnaese K, Mummery R, Gundelfinger E D, Beesley P W. FEBS Lett. 1998;429:284–288. doi: 10.1016/s0014-5793(98)00616-4. [DOI] [PubMed] [Google Scholar]
- 27.Smalla K H, Angenstein F, Richter K, Gundelfinger E D, Staak S. NeuroReport. 1998;9:813–817. doi: 10.1097/00001756-199803300-00009. [DOI] [PubMed] [Google Scholar]
- 28.Morgan D G, Routtenberg A. Brain Res. 1979;179:343–354. doi: 10.1016/0006-8993(79)90449-9. [DOI] [PubMed] [Google Scholar]
- 29.Jork R, Grecksch G, Matthies H. Pharmacol Biochem Behav. 1986;25:1137–1144. doi: 10.1016/0091-3057(86)90100-0. [DOI] [PubMed] [Google Scholar]
- 30.Bullock S, Rose S P R, Zamani R. J Neurochem. 1992;58:2145–2154. doi: 10.1111/j.1471-4159.1992.tb10957.x. [DOI] [PubMed] [Google Scholar]
- 31.Krug M, Jork R, Reymann K, Wagner M, Matthies H. Brain Res. 1991;540:237–242. doi: 10.1016/0006-8993(91)90513-u. [DOI] [PubMed] [Google Scholar]
- 32.Matthies H, Jr, Kretlow J, Matthies H, Smalla K H, Staak S, Krug M. Neuroscience. 1999;91:175–183. doi: 10.1016/s0306-4522(98)00628-9. [DOI] [PubMed] [Google Scholar]
- 33.Mayer U, Nischt R, Pöschl E, Mann K, Fukuda K, Gerl M, Yamada Y, Timpl R. EMBO J. 1993;12:1879–1885. doi: 10.1002/j.1460-2075.1993.tb05836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boeckers T M, Kreutz M R, Winter C, Zuschratter W, Smalla K H, Sanmartí-Vila L, Wex H, Langnaese K, Bockmann J, Garner C C, Gundelfinger E D. J Neurosci. 1999;19:6506–6518. doi: 10.1523/JNEUROSCI.19-15-06506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nedivi E, Hevroni D, Naot D, Israeli D, Citri Y. Nature (London) 1993;363:718–722. doi: 10.1038/363718a0. [DOI] [PubMed] [Google Scholar]
- 36.Carlin R K, Grab D J, Cohen R S, Siekievitz P. J Cell Biol. 1980;86:831–843. doi: 10.1083/jcb.86.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.tom Dieck S, Sanmarti-Vila L, Langnaese K, Richter K, Kindler S, Soyke A, Wex H, Smalla K H, Kampf U, Franzer J T, et al. J Cell Biol. 1998;142:499–509. doi: 10.1083/jcb.142.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Angenstein F, Matthies H, Jr, Staeck S, Reymann K G, Staak S. Neurochem Int. 1992;21:403–408. doi: 10.1016/0197-0186(92)90191-s. [DOI] [PubMed] [Google Scholar]
- 39.Kuhn T B, Stoeckli E T, Condrau M A, Rathjen F G, Sonderegger P. J Cell Biol. 1991;115:1113–1126. doi: 10.1083/jcb.115.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Amaral D G, Witter M P. In: The Rat Nervous System. Paxinos G, editor. London: Academic; 1995. pp. 443–494. [Google Scholar]
- 41.Nadler J V, Perry B W, Cotman C W. Nature (London) 1978;271:676–677. doi: 10.1038/271676a0. [DOI] [PubMed] [Google Scholar]
- 42.Sperk G. Prog Neurobiol. 1994;42:1–32. doi: 10.1016/0301-0082(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 43.Ranheim T S, Edelman G M, Cunningham B A. Proc Natl Acad Sci USA. 1996;93:4071–4075. doi: 10.1073/pnas.93.9.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lemmon V, Farr K L, Lagenaur C. Neuron. 1989;2:1597–1603. doi: 10.1016/0896-6273(89)90048-2. [DOI] [PubMed] [Google Scholar]
- 45.Hu B R, Park M, Martone M E, Fischer W H, Ellisman M H, Zivin J A. J Neurosci. 1998;18:625–633. doi: 10.1523/JNEUROSCI.18-02-00625.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martone M E, Jones Y Z, Young S J, Ellisman M H, Zivin J A, Hu B R. J Neurosci. 1999;19:1988–1997. doi: 10.1523/JNEUROSCI.19-06-01988.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schuster T, Krug M, Wenzel J. Brain Res. 1990;523:171–174. doi: 10.1016/0006-8993(90)91654-y. [DOI] [PubMed] [Google Scholar]
- 48.Buchs P A, Muller D. Proc Natl Acad Sci USA. 1996;93:8040–8045. doi: 10.1073/pnas.93.15.8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geinisman Y, Detoledo-Morrell L, Morrell F, Persina I S, Beatty M A. J Comp Neurol. 1996;368:413–423. doi: 10.1002/(SICI)1096-9861(19960506)368:3<413::AID-CNE7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 50.Gray G. In: Encyclopedia of Neuroscience. Adelman G, editor. II. Boston: Birkhäuser; 1987. pp. 1158–1162. [Google Scholar]
- 51.Muller D, Wang C, Skibo G, Toni N, Cremer H, Calaora V, Rougon G, Kiss J Z. Neuron. 1996;17:413–422. doi: 10.1016/s0896-6273(00)80174-9. [DOI] [PubMed] [Google Scholar]
- 52.Nalbantoglu J, Tirado-Santiago G, Lahsaini A, Poirier J, Goncalves O, Verge G, Momoli F, Welner S A, Massicotte G, Julien J P, Shapiro M L. Nature (London) 1997;387:500–505. doi: 10.1038/387500a0. [DOI] [PubMed] [Google Scholar]
- 53.Seabrook G R, Smith D W, Bowery B J, Easter A, Reynolds T, Fitzjohn S M, Morton R A, Zheng H, Dawson G R, Sirinathsinghji D J, et al. Neuropharmacology. 1999;38:349–359. doi: 10.1016/s0028-3908(98)00204-4. [DOI] [PubMed] [Google Scholar]
- 54.Lauri S E, Kaukinen S, Kinnunen T, Ylinen A, Imai S, Kaila K, Taira T, Rauvala H. J Neurosci. 1999;19:1226–1235. doi: 10.1523/JNEUROSCI.19-04-01226.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reymann K G, Frey U, Jork R, Matthies H. Brain Res. 1988;440:305–314. doi: 10.1016/0006-8993(88)91000-1. [DOI] [PubMed] [Google Scholar]
- 56.Colley P A, Sheu F S, Routtenberg A. J Neurosci. 1990;10:3353–3360. doi: 10.1523/JNEUROSCI.10-10-03353.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kose A, Ito A, Saito N, Tanaka C. Brain Res. 1990;518:209–217. doi: 10.1016/0006-8993(90)90974-g. [DOI] [PubMed] [Google Scholar]
- 58.Meiri K F, Saffell J L, Walsh F S, Doherty P. J Neurosci. 1998;18:10429–10437. doi: 10.1523/JNEUROSCI.18-24-10429.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Engert F, Bonhoeffer T. Nature (London) 1999;399:66–70. doi: 10.1038/19978. [DOI] [PubMed] [Google Scholar]
- 60.Maletic-Savatic M, Malinow R, Svoboda K. Science. 1999;283:1923–1926. doi: 10.1126/science.283.5409.1923. [DOI] [PubMed] [Google Scholar]
- 61.Toni N, Buchs P A, Nikonenko I, Bron C R, Muller D. Nature (London) 1999;402:421–425. doi: 10.1038/46574. [DOI] [PubMed] [Google Scholar]
- 62.Itoh K, Stevens B, Schachner M, Fields R D. Science. 1995;270:1369–1372. doi: 10.1126/science.270.5240.1369. [DOI] [PubMed] [Google Scholar]
- 63.Itoh K, Ozaki M, Stevens B, Fields R D. J Neurobiol. 1997;33:735–748. doi: 10.1002/(sici)1097-4695(19971120)33:6<735::aid-neu3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 64.Nakic M, Manahan-Vaughan D, Reymann K G, Schachner M. J Neurobiol. 1998;37:393–404. [PubMed] [Google Scholar]
- 65.Schuster T, Krug M, Hassan H, Schachner M. J Neurobiol. 1998;37:359–372. doi: 10.1002/(sici)1097-4695(19981115)37:3<359::aid-neu2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]