Abstract
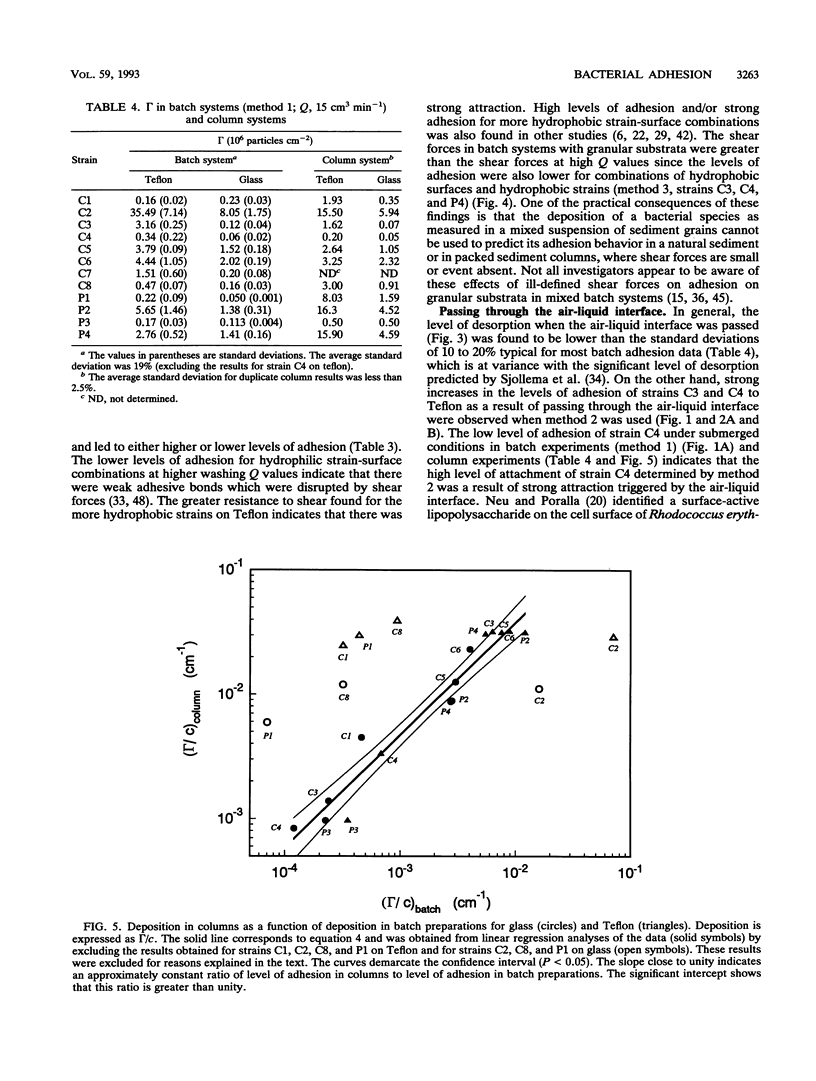
The deposition of various pseudomonads and coryneform bacteria with different hydrophobicities (water contact angles) and negative cell surface charges on negatively charged Teflon and glass surfaces was investigated. The levels of deposition varied between 5.0 × 104 and 1.6 × 107 cells cm-2 and between 5.0 × 104 and 3.6 × 107 cells cm-2 for dynamic column and static batch systems, respectively, indicating that there was a wide variation in physicochemical interactions. Batch and column results were compared in order to better distinguish between hydrodynamic and other system-dependent influences and method-independent physicochemical interactions. Despite the shorter suspension-solid contact time in columns (1 h) than in batch systems (4 h), the level of deposition (expressed as the number of cells that adhered) divided by the applied ambient cell concentration was 4.12 ± 1.63 times higher in columns than in batch sytems for 15 of 22 strain-surface combinations studied. This demonstrates that transport of microbial particles from bulk liquid to surfaces is more efficient in dynamic columns (transport dominated by convection and diffusion) than in static batch systems (transport by diffusion only). The relative constancy of this ratio for the 15 combinations shows that physicochemical interactions affect adhesion similarly in the two systems. The deviating deposition behavior of the other seven strain-surface combinations could be attributed to method-dependent effects resulting from specific cell characteristics (e.g., to the presence of capsular polymers, to an ability to aggregate, to large cell sizes, or to a tendency to desorb after passage through an air-liquid interface).
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Absolom D. R., Lamberti F. V., Policova Z., Zingg W., van Oss C. J., Neumann A. W. Surface thermodynamics of bacterial adhesion. Appl Environ Microbiol. 1983 Jul;46(1):90–97. doi: 10.1128/aem.46.1.90-97.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendinger B., Kroppenstedt R. M., Klatte S., Altendorf K. Chemotaxonomic differentiation of coryneform bacteria isolated from biofilters. Int J Syst Bacteriol. 1992 Jul;42(3):474–486. doi: 10.1099/00207713-42-3-474. [DOI] [PubMed] [Google Scholar]
- Busscher H. J., Weerkamp A. H., van der Mei H. C., van Pelt A. W., de Jong H. P., Arends J. Measurement of the surface free energy of bacterial cell surfaces and its relevance for adhesion. Appl Environ Microbiol. 1984 Nov;48(5):980–983. doi: 10.1128/aem.48.5.980-983.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn E., Hellwig M., Reineke W., Knackmuss H. J. Isolation and characterization of a 3-chlorobenzoate degrading pseudomonad. Arch Microbiol. 1974;99(1):61–70. doi: 10.1007/BF00696222. [DOI] [PubMed] [Google Scholar]
- Jang L. K., Chang P. W., Findley J. E., Yen T. F. Selection of bacteria with favorable transport properties through porous rock for the application of microbial-enhanced oil recovery. Appl Environ Microbiol. 1983 Nov;46(5):1066–1072. doi: 10.1128/aem.46.5.1066-1072.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraa G., Bethe B. M., van Neerven A. R., Van den Tweel W. J., Van der Wende E., Zehnder A. J. Degradation 1,2-dimethylbenzene by Corynebacterium strain C125. Antonie Van Leeuwenhoek. 1987;53(3):159–170. doi: 10.1007/BF00393844. [DOI] [PubMed] [Google Scholar]
- Schraa G., Boone M. L., Jetten M. S., van Neerven A. R., Colberg P. J., Zehnder A. J. Degradation of 1,4-dichlorobenzene by Alcaligenes sp. strain A175. Appl Environ Microbiol. 1986 Dec;52(6):1374–1381. doi: 10.1128/aem.52.6.1374-1381.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevors J. T., van Elsas J. D., van Overbeek L. S., Starodub M. E. Transport of a genetically engineered Pseudomonas fluorescens strain through a soil microcosm. Appl Environ Microbiol. 1990 Feb;56(2):401–408. doi: 10.1128/aem.56.2.401-408.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. A., Worsey M. J. Ubiquity of plasmids in coding for toluene and xylene metabolism in soil bacteria: evidence for the existence of new TOL plasmids. J Bacteriol. 1976 Mar;125(3):818–828. doi: 10.1128/jb.125.3.818-828.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witholt B., de Smet M. J., Kingma J., van Beilen J. B., Kok M., Lageveen R. G., Eggink G. Bioconversions of aliphatic compounds by Pseudomonas oleovorans in multiphase bioreactors: background and economic potential. Trends Biotechnol. 1990 Feb;8(2):46–52. doi: 10.1016/0167-7799(90)90133-i. [DOI] [PubMed] [Google Scholar]
- Xia Z., Woo L., van de Ven T. G. Microrheological aspects of adhesion of Escherichia coli on glass. Biorheology. 1989;26(2):359–375. doi: 10.3233/bir-1989-26219. [DOI] [PubMed] [Google Scholar]
- van Loosdrecht M. C., Lyklema J., Norde W., Schraa G., Zehnder A. J. Electrophoretic mobility and hydrophobicity as a measured to predict the initial steps of bacterial adhesion. Appl Environ Microbiol. 1987 Aug;53(8):1898–1901. doi: 10.1128/aem.53.8.1898-1901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loosdrecht M. C., Lyklema J., Norde W., Schraa G., Zehnder A. J. The role of bacterial cell wall hydrophobicity in adhesion. Appl Environ Microbiol. 1987 Aug;53(8):1893–1897. doi: 10.1128/aem.53.8.1893-1897.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Pelt A. W., Weerkamp A. H., Uyen M. H., Busscher H. J., de Jong H. P., Arends J. Adhesion of Streptococcus sanguis CH3 to polymers with different surface free energies. Appl Environ Microbiol. 1985 May;49(5):1270–1275. doi: 10.1128/aem.49.5.1270-1275.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]