Abstract
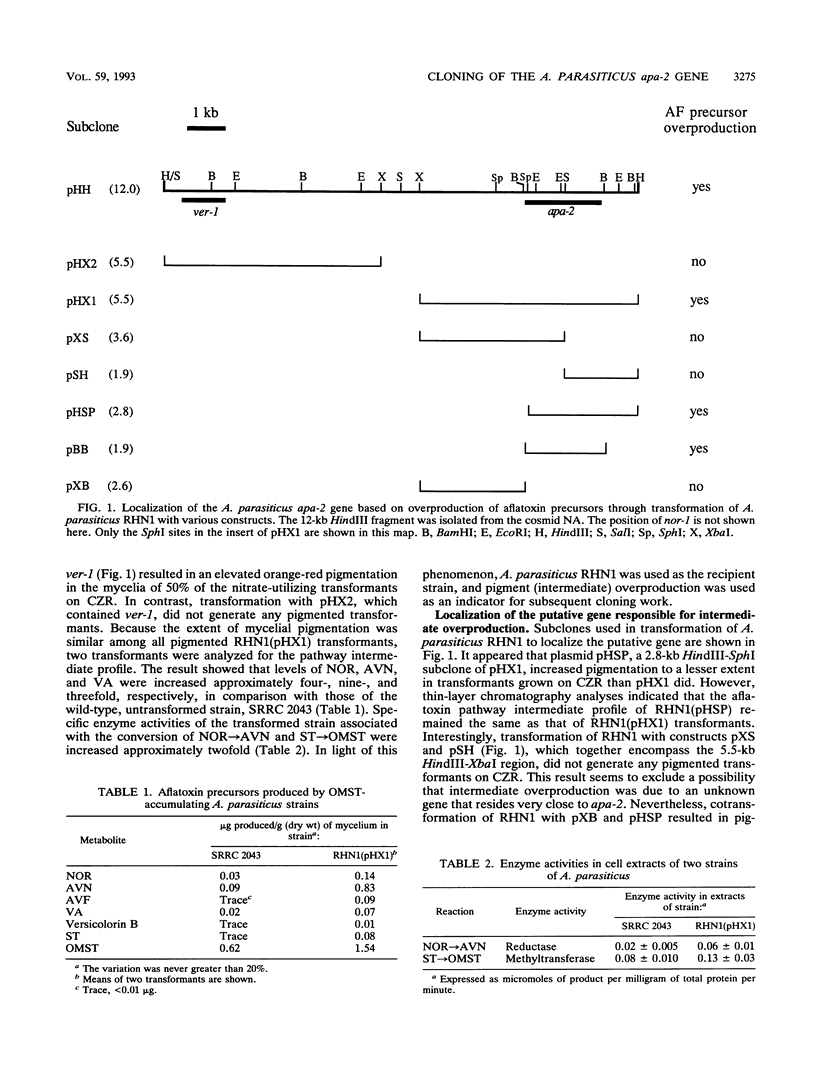
An Aspergillus parasiticus gene, designated apa-2, was identified as a regulatory gene associated with aflatoxin biosynthesis. The apa-2 gene was cloned on the basis of overproduction of pathway intermediates following transformation of fungal strains with cosmid DNA containing the aflatoxin biosynthetic genes nor-1 and ver-1. Transformation of an O-methylsterigmatocystin-accumulating strain, A. parasiticus SRRC 2043, with a 5.5-kb HindIII-XbaI DNA fragment containing apa-2 resulted in overproduction of all aflatoxin pathway intermediates analyzed. Specific enzyme activities associated with the conversion of norsolorinic acid and sterigmatocystin were increased approximately twofold. The apa-2 gene was found to complement an A. flavus afl-2 mutant strain for aflatoxin production, suggesting that apa-2 is functionally homologous to afl-2. Comparison of the A. parasiticus apa-2 gene DNA sequence with that of the A. flavus afl-2 gene (G. A. Payne, G. J. Nystorm, D. Bhatnagar, T. E. Cleveland, and C. P. Woloshuk, Appl. Environ. Microbiol. 59:156-162, 1993) showed that they shared > 95% DNA homology. Physical mapping of cosmid subclones placed apa-2 approximately 8 kb from ver-1.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADYE J., MATELES R. I. INCORPORATION OF LABELLED COMPOUNDS INTO AFLATOXINS. Biochim Biophys Acta. 1964 May 11;86:418–420. doi: 10.1016/0304-4165(64)90077-7. [DOI] [PubMed] [Google Scholar]
- Andrianopoulos A., Hynes M. J. Sequence and functional analysis of the positively acting regulatory gene amdR from Aspergillus nidulans. Mol Cell Biol. 1990 Jun;10(6):3194–3203. doi: 10.1128/mcb.10.6.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. W. Aflatoxins and anthraquinones from diploids of Aspergillus parasiticus. J Gen Microbiol. 1979 Jul;113(1):127–136. doi: 10.1099/00221287-113-1-127. [DOI] [PubMed] [Google Scholar]
- Bennett J. W., Rubin P. L., Lee L. S., Chen P. N. Influence of trace elements and nitrogen sources on versicolorin production by a mutant strain of Aspergillus parasiticus. Mycopathologia. 1979 Dec 28;69(3):161–166. doi: 10.1007/BF00452829. [DOI] [PubMed] [Google Scholar]
- Bhatnagar D., McCormick S. P., Lee L. S., Hill R. A. Identification of O-methylsterigmatocystin as an aflatoxin B1 and G1 precursor in Aspergillus parasiticus. Appl Environ Microbiol. 1987 May;53(5):1028–1033. doi: 10.1128/aem.53.5.1028-1033.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressac B., Kew M., Wands J., Ozturk M. Selective G to T mutations of p53 gene in hepatocellular carcinoma from southern Africa. Nature. 1991 Apr 4;350(6317):429–431. doi: 10.1038/350429a0. [DOI] [PubMed] [Google Scholar]
- Challen C., Lunec J., Warren W., Collier J., Bassendine M. F. Analysis of the p53 tumor-suppressor gene in hepatocellular carcinomas from Britain. Hepatology. 1992 Dec;16(6):1362–1366. doi: 10.1002/hep.1840160610. [DOI] [PubMed] [Google Scholar]
- Chang P. K., Skory C. D., Linz J. E. Cloning of a gene associated with aflatoxin B1 biosynthesis in Aspergillus parasiticus. Curr Genet. 1992 Mar;21(3):231–233. doi: 10.1007/BF00336846. [DOI] [PubMed] [Google Scholar]
- Cleveland T. E., Bhatnagar D., Brown R. L. Aflatoxin production via cross-feeding of pathway intermediates during cofermentation of aflatoxin pathway-blocked Aspergillus parasiticus mutants. Appl Environ Microbiol. 1991 Oct;57(10):2907–2911. doi: 10.1128/aem.57.10.2907-2911.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland T. E., Bhatnagar D. Evidence for de novo synthesis of an aflatoxin pathway methyltransferase near the cessation of active growth and the onset of aflatoxin biosynthesis in Aspergillus parasiticus mycelia. Can J Microbiol. 1990 Jan;36(1):1–5. doi: 10.1139/m90-001. [DOI] [PubMed] [Google Scholar]
- Cove D. J. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim Biophys Acta. 1966 Jan 11;113(1):51–56. doi: 10.1016/s0926-6593(66)80120-0. [DOI] [PubMed] [Google Scholar]
- Davis N. D., Iyer S. K., Diener U. L. Improved method of screening for aflatoxin with a coconut agar medium. Appl Environ Microbiol. 1987 Jul;53(7):1593–1595. doi: 10.1128/aem.53.7.1593-1595.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton M. F. Enzymes and aflatoxin biosynthesis. Microbiol Rev. 1988 Jun;52(2):274–295. doi: 10.1128/mr.52.2.274-295.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M., Hollenberg S. M. Zinc fingers: gilt by association. Cell. 1988 Jan 15;52(1):1–3. doi: 10.1016/0092-8674(88)90522-3. [DOI] [PubMed] [Google Scholar]
- Feng G. H., Chu F. S., Leonard T. J. Molecular cloning of genes related to aflatoxin biosynthesis by differential screening. Appl Environ Microbiol. 1992 Feb;58(2):455–460. doi: 10.1128/aem.58.2.455-460.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horng J. S., Chang P. K., Pestka J. J., Linz J. E. Development of a homologous transformation system for Aspergillus parasiticus with the gene encoding nitrate reductase. Mol Gen Genet. 1990 Nov;224(2):294–296. doi: 10.1007/BF00271564. [DOI] [PubMed] [Google Scholar]
- Jelinek C. F., Pohland A. E., Wood G. E. Worldwide occurrence of mycotoxins in foods and feeds--an update. J Assoc Off Anal Chem. 1989 Mar-Apr;72(2):223–230. [PubMed] [Google Scholar]
- Johnston M. A model fungal gene regulatory mechanism: the GAL genes of Saccharomyces cerevisiae. Microbiol Rev. 1987 Dec;51(4):458–476. doi: 10.1128/mr.51.4.458-476.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller N. P., Dischinger H. C., Jr, Bhatnagar D., Cleveland T. E., Ullah A. H. Purification of a 40-kilodalton methyltransferase active in the aflatoxin biosynthetic pathway. Appl Environ Microbiol. 1993 Feb;59(2):479–484. doi: 10.1128/aem.59.2.479-484.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepom P., Kloss H. Production of sterigmatocystin by Aspergillus versicolor isolated from roughage. Mycopathologia. 1988 Jan;101(1):25–29. doi: 10.1007/BF00455665. [DOI] [PubMed] [Google Scholar]
- Lin B. K., Anderson J. A. Purification and properties of versiconal cyclase from Aspergillus parasiticus. Arch Biochem Biophys. 1992 Feb 14;293(1):67–70. doi: 10.1016/0003-9861(92)90366-5. [DOI] [PubMed] [Google Scholar]
- Linz J. E., Pestka J. J. Mycotoxins: molecular strategies for control. Biotechnology. 1992;23:217–231. [PubMed] [Google Scholar]
- Mayorga M. E., Timberlake W. E. The developmentally regulated Aspergillus nidulans wA gene encodes a polypeptide homologous to polyketide and fatty acid synthases. Mol Gen Genet. 1992 Nov;235(2-3):205–212. doi: 10.1007/BF00279362. [DOI] [PubMed] [Google Scholar]
- Niehaus W. G., Jr, Jiang W. P. Nitrate induces enzymes of the mannitol cycle and suppresses versicolorin synthesis in Aspergillus parasiticus. Mycopathologia. 1989 Sep;107(2-3):131–137. doi: 10.1007/BF00707550. [DOI] [PubMed] [Google Scholar]
- Payne G. A., Nystrom G. J., Bhatnagar D., Cleveland T. E., Woloshuk C. P. Cloning of the afl-2 gene involved in aflatoxin biosynthesis from Aspergillus flavus. Appl Environ Microbiol. 1993 Jan;59(1):156–162. doi: 10.1128/aem.59.1.156-162.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler A. F., Palmer J. G., Eisenberg W. V. Aflatoxin Production by Aspergillus flavus as Related to Various Temperatures. Appl Microbiol. 1967 Sep;15(5):1006–1009. doi: 10.1128/am.15.5.1006-1009.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skory C. D., Chang P. K., Cary J., Linz J. E. Isolation and characterization of a gene from Aspergillus parasiticus associated with the conversion of versicolorin A to sterigmatocystin in aflatoxin biosynthesis. Appl Environ Microbiol. 1992 Nov;58(11):3527–3537. doi: 10.1128/aem.58.11.3527-3537.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skory C. D., Chang P. K., Linz J. E. Regulated expression of the nor-1 and ver-1 genes associated with aflatoxin biosynthesis. Appl Environ Microbiol. 1993 May;59(5):1642–1646. doi: 10.1128/aem.59.5.1642-1646.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skory C. D., Horng J. S., Pestka J. J., Linz J. E. Transformation of Aspergillus parasiticus with a homologous gene (pyrG) involved in pyrimidine biosynthesis. Appl Environ Microbiol. 1990 Nov;56(11):3315–3320. doi: 10.1128/aem.56.11.3315-3320.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woloshuk C. P., Seip E. R., Payne G. A., Adkins C. R. Genetic transformation system for the aflatoxin-producing fungus Aspergillus flavus. Appl Environ Microbiol. 1989 Jan;55(1):86–90. doi: 10.1128/aem.55.1.86-90.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


