Abstract
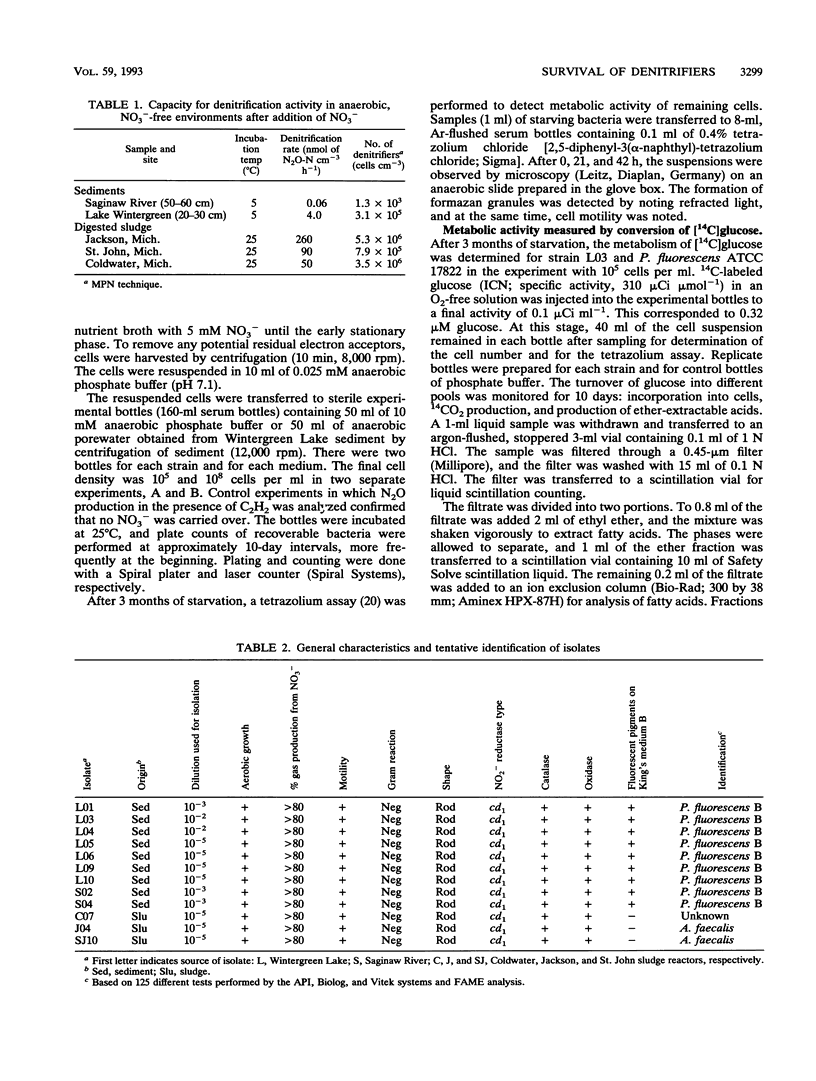
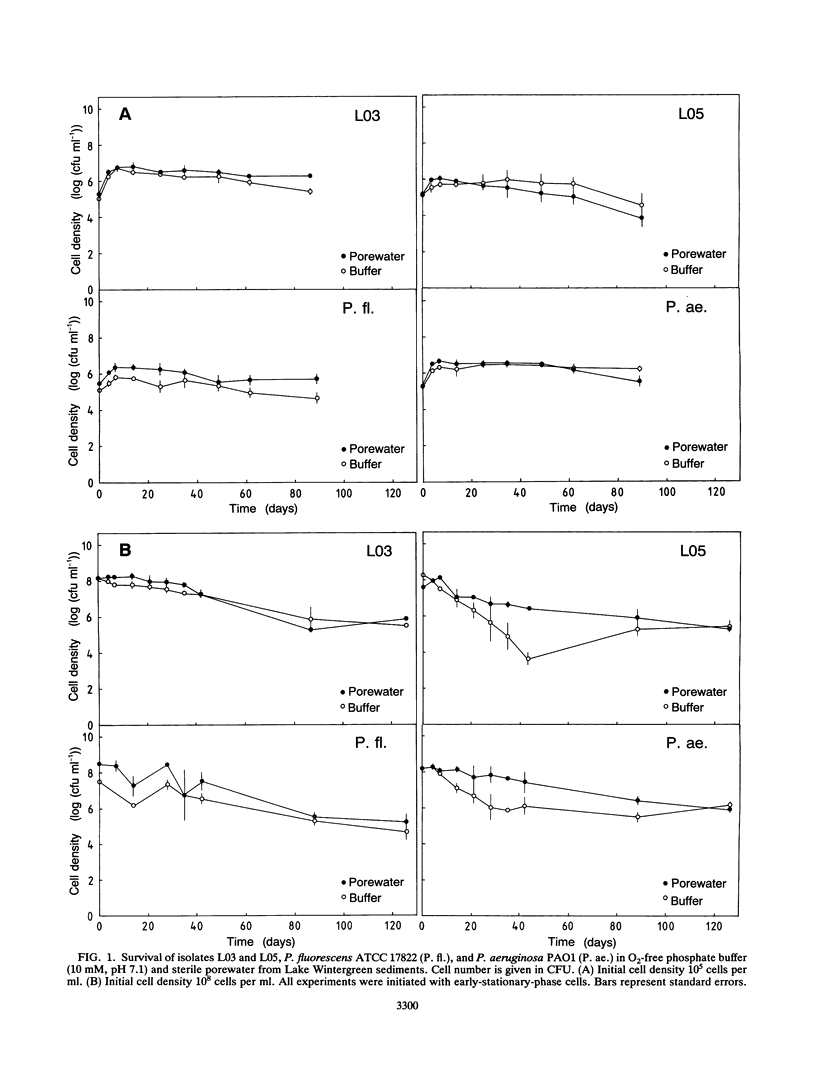
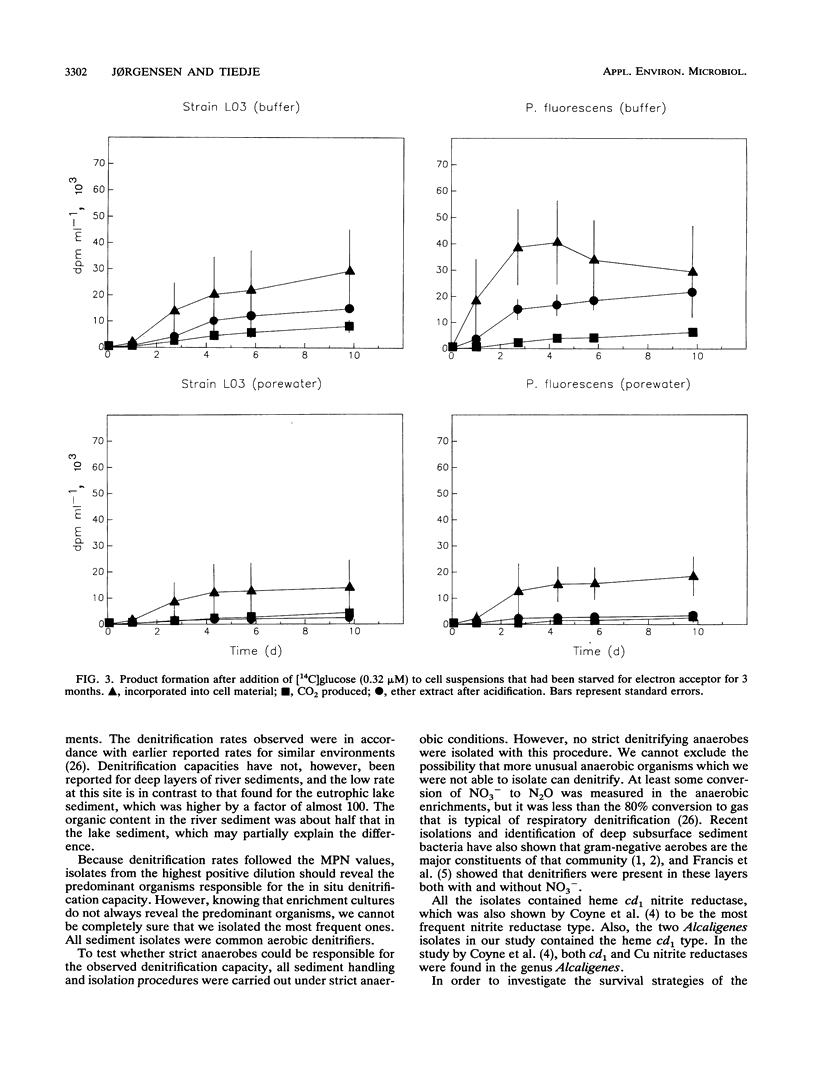
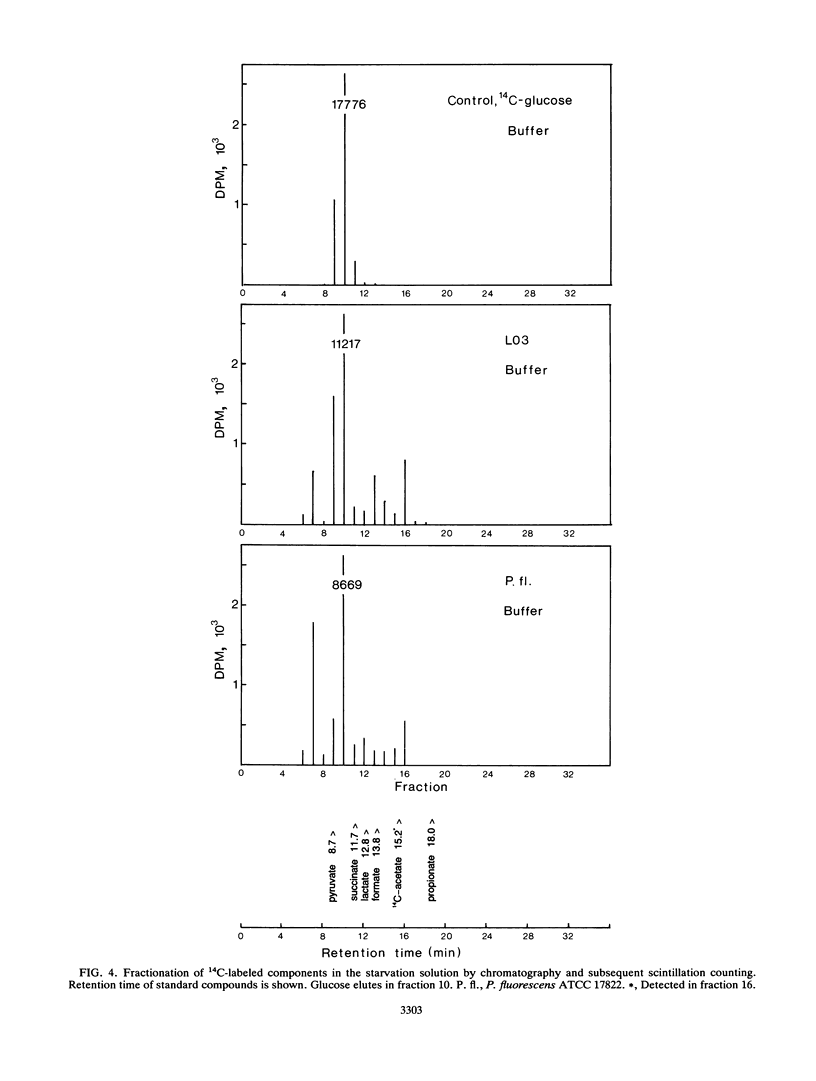
Experiments were undertaken to explain the occurrence of a high denitrification capacity in anaerobic, NO3--free habitats. Deep layers of freshwater sediments that were buried more than 40 years ago and digested sludge were the habitats studied. The denitrifier populations were 3.1 × 103 and 3.1 × 105 cells cm-3 in deep sediments from a river and lake, respectively, and 5.3 × 106 cells cm-3 in digested sludge. The denitrification capacities of the samples reflected the population densities. Strict anaerobic procedures were used to obtain the predominant isolates that would grow on anaerobic medium with NO3-. All strict anaerobes isolated failed to denitrify. All isolates that denitrified were aerobic, gram-negative bacteria, particularly species of Pseudomonas and Alcaligenes. No detectable growth was observed when these strains were incubated with electron acceptors other than NO3- or O2. When representative isolates were added to sterile, O2- and NO3--free porewater from their original locations at their natural densities (105 cells cm-3), no change in viable population was noted over 3 months of incubation. Metabolic activity was demonstrated in these cells by slow formation of formazan granules when exposed to tetrazolium and by observation of motile cells. When [14C]glucose was added to cell suspensions of the pseudomonads that had been starved for 3 months without electron acceptors (O2 or NO3-), 14C-labeled products, including cell biomass, 14CO2, and fermentation products, were produced. The high denitrification capacity of these anaerobic environments appears to be due to conventional respiratory denitrifiers. These organisms have the capacity for long-term survival without O2 or NO3- and appear to be capable of providing for their maintenance by carrying on a low level of fermentation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balkwill D. L., Fredrickson J. K., Thomas J. M. Vertical and horizontal variations in the physiological diversity of the aerobic chemoheterotrophic bacterial microflora in deep southeast coastal plain subsurface sediments. Appl Environ Microbiol. 1989 May;55(5):1058–1065. doi: 10.1128/aem.55.5.1058-1065.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne M. S., Arunakumari A., Averill B. A., Tiedje J. M. Immunological identification and distribution of dissimilatory heme cd1 and nonheme copper nitrite reductases in denitrifying bacteria. Appl Environ Microbiol. 1989 Nov;55(11):2924–2931. doi: 10.1128/aem.55.11.2924-2931.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galimand M., Gamper M., Zimmermann A., Haas D. Positive FNR-like control of anaerobic arginine degradation and nitrate respiration in Pseudomonas aeruginosa. J Bacteriol. 1991 Mar;173(5):1598–1606. doi: 10.1128/jb.173.5.1598-1606.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble T. N., Betlach M. R., Tiedje J. M. Numerically dominant denitrifying bacteria from world soils. Appl Environ Microbiol. 1977 Apr;33(4):926–939. doi: 10.1128/aem.33.4.926-939.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen K. S. Annual pattern of denitrification and nitrate ammonification in estuarine sediment. Appl Environ Microbiol. 1989 Jul;55(7):1841–1847. doi: 10.1128/aem.55.7.1841-1847.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar H. F., Tiedje J. M., Firestone R. B. Denitrification and dissimilatory nitrate reduction to ammonium in digested sludge. Can J Microbiol. 1981 Sep;27(9):878–885. doi: 10.1139/m81-139. [DOI] [PubMed] [Google Scholar]
- Molongoski J. J., Klug M. J. Characterization of anaerobic heterotrophic bacteria isolated from freshwater lake sediments. Appl Environ Microbiol. 1976 Jan;31(1):83–90. doi: 10.1128/aem.31.1.83-90.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Umberger C., Culbertson C. W., Smith R. L. Denitrification in san francisco bay intertidal sediments. Appl Environ Microbiol. 1984 May;47(5):1106–1112. doi: 10.1128/aem.47.5.1106-1112.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. B., Tiedje J. M. Isolation and characterization of a nitrite reductase gene and its use as a probe for denitrifying bacteria. Appl Environ Microbiol. 1992 Jan;58(1):376–384. doi: 10.1128/aem.58.1.376-384.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen J. Capacity for denitrification and reduction of nitrate to ammonia in a coastal marine sediment. Appl Environ Microbiol. 1978 Feb;35(2):301–305. doi: 10.1128/aem.35.2.301-305.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann A., Reimmann C., Galimand M., Haas D. Anaerobic growth and cyanide synthesis of Pseudomonas aeruginosa depend on anr, a regulatory gene homologous with fnr of Escherichia coli. Mol Microbiol. 1991 Jun;5(6):1483–1490. doi: 10.1111/j.1365-2958.1991.tb00794.x. [DOI] [PubMed] [Google Scholar]



