Abstract
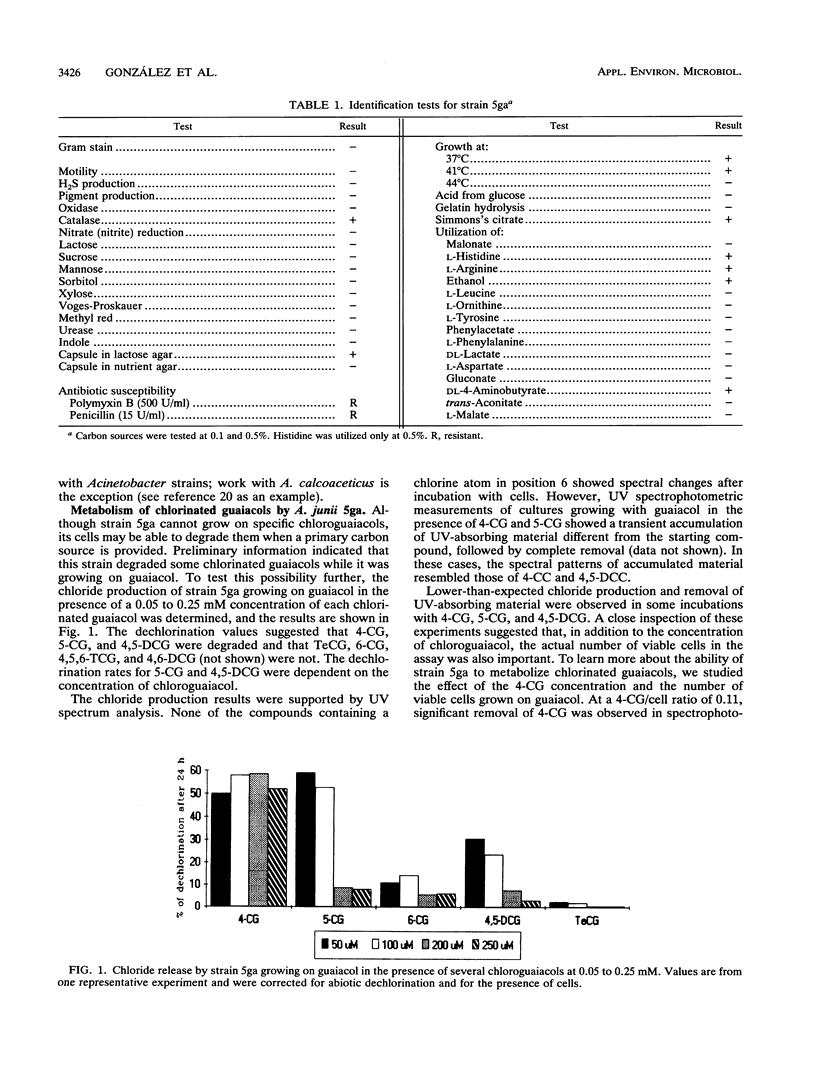
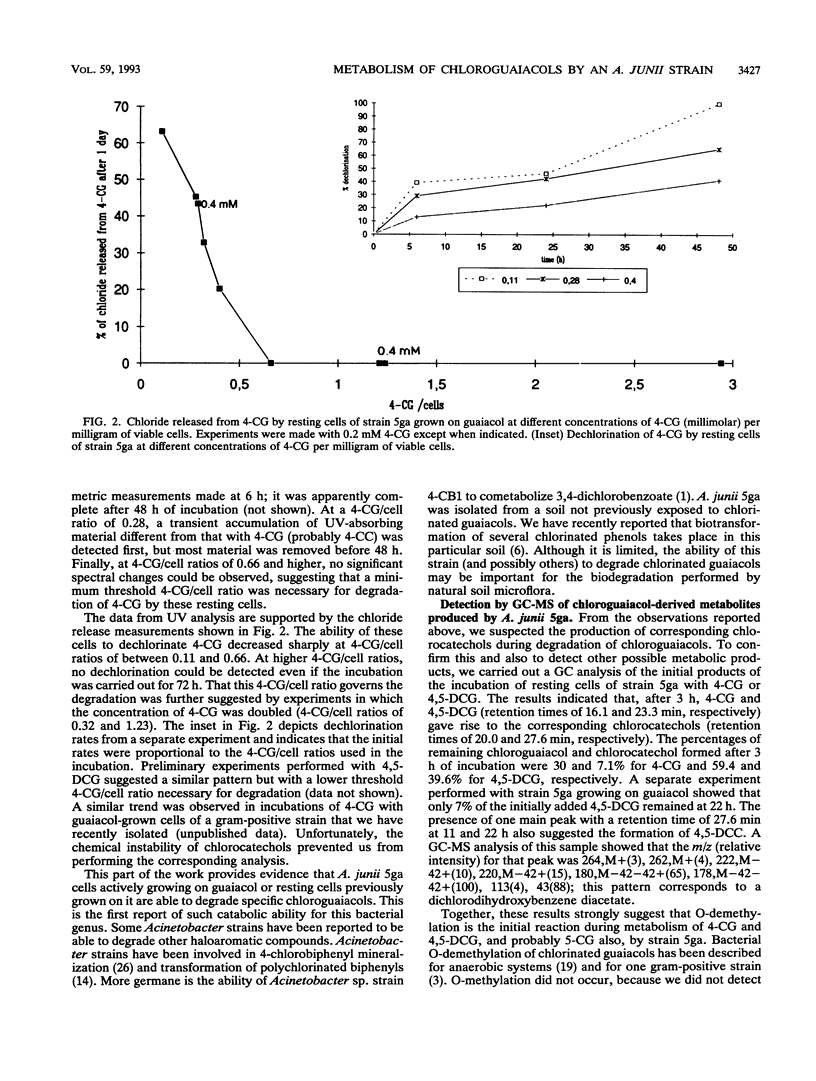
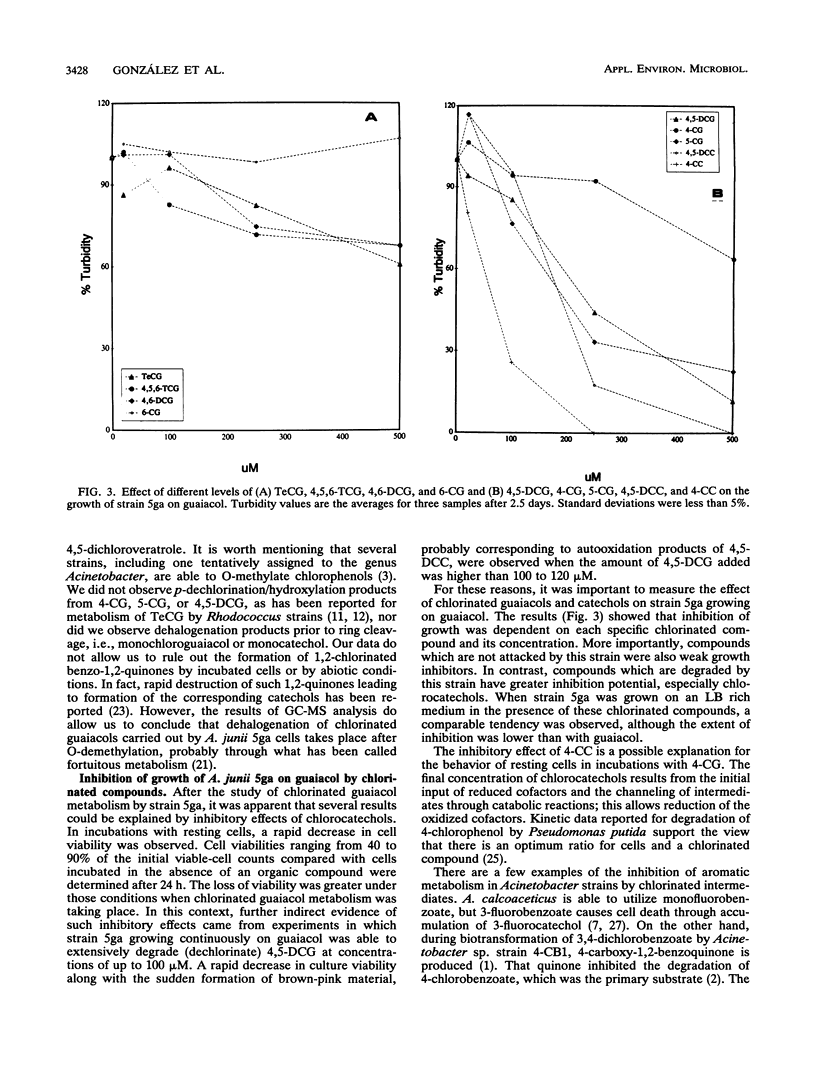
The metabolism of chlorinated guaiacols by a pure bacterial strain identified by its ability to use guaiacol as the sole carbon and energy source was studied. This strain, identified as Acinetobacter junii 5ga, was unable to grow on several chlorinated guaiacols and catechols. However, strain 5ga grown on guaiacol degraded 4- and 5-chloroguaiacol and 4,5-dichloroguaiacol. Under the same conditions, these cells did not degrade 6-chloroguaiacol, 4,6-dichloroguaiacol, 4,5,6-trichloroguaiacol, or tetrachloroguaiacol, suggesting that the substitution at the 6 position in the ring prevents metabolism of the compound. Degradation of 4-chloroguaiacol was dependent on the initial ratio between the chlorinated compound and viable cells. Transient formation of chlorocatechols resulting from incubation of cells with 4-chloroguaiacol or 4,5-dichloroguaiacol was suggested by UV spectroscopy. Gas chromatography analyses of samples from cultures of strain 5ga grown on guaiacol and incubated with 4- and 4,5-dichloroguaiacol confirmed the presence of 4-chlorocatechol and 4,5-dichlorocatechol, respectively. The formation of the latter was corroborated by gas chromatography-mass spectrometry. Thus, this strain is able to initiate metabolism of specific chlorinated guaiacols by O-demethylation. The starting chlorinated guaiacols and their O-demethylated metabolites inhibited the growth of A. junii 5ga on guaiacol.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adriaens P., Focht D. D. Cometabolism of 3,4-dichlorobenzoate by Acinetobacter sp. strain 4-CB1. Appl Environ Microbiol. 1991 Jan;57(1):173–179. doi: 10.1128/aem.57.1.173-179.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard A. S., Remberger M., Neilson A. H. Bacterial o-methylation of chloroguaiacols: effect of substrate concentration, cell density, and growth conditions. Appl Environ Microbiol. 1985 Feb;49(2):279–288. doi: 10.1128/aem.49.2.279-288.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke K. F., Callely A. G., Livingstone A., Fewson C. A. Metabolism of monofluorobenzoates by Acinetobacter calcoaceticus N.C.I.B. 8250. Formation of monofluorocatechols. Biochim Biophys Acta. 1975 Oct 9;404(2):169–179. doi: 10.1016/0304-4165(75)90323-2. [DOI] [PubMed] [Google Scholar]
- Higson F. K., Focht D. D. Degradation of 2-methylbenzoic acid by Pseudomonas cepacia MB2. Appl Environ Microbiol. 1992 Jan;58(1):194–200. doi: 10.1128/aem.58.1.194-200.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häggblom M. M., Apajalahti J. H., Salkinoja-Salonen M. S. O-Methylation of Chlorinated para-Hydroquinones by Rhodococcus chlorophenolicus. Appl Environ Microbiol. 1988 Jul;54(7):1818–1824. doi: 10.1128/aem.54.7.1818-1824.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häggblom M. M., Janke D., Salkinoja-Salonen M. S. Hydroxylation and Dechlorination of Tetrachlorohydroquinone by Rhodococcus sp. Strain CP-2 Cell Extracts. Appl Environ Microbiol. 1989 Feb;55(2):516–519. doi: 10.1128/aem.55.2.516-519.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häggblom M. M. Microbial breakdown of halogenated aromatic pesticides and related compounds. FEMS Microbiol Rev. 1992 Sep;9(1):29–71. doi: 10.1111/j.1574-6968.1992.tb05823.x. [DOI] [PubMed] [Google Scholar]
- Häggblom M. M., Nohynek L. J., Salkinoja-Salonen M. S. Degradation and O-methylation of chlorinated phenolic compounds by Rhodococcus and Mycobacterium strains. Appl Environ Microbiol. 1988 Dec;54(12):3043–3052. doi: 10.1128/aem.54.12.3043-3052.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler H. P., Kohler-Staub D., Focht D. D. Cometabolism of polychlorinated biphenyls: enhanced transformation of Aroclor 1254 by growing bacterial cells. Appl Environ Microbiol. 1988 Aug;54(8):1940–1945. doi: 10.1128/aem.54.8.1940-1945.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröckel L., Focht D. D. Construction of chlorobenzene-utilizing recombinants by progenitive manifestation of a rare event. Appl Environ Microbiol. 1987 Oct;53(10):2470–2475. doi: 10.1128/aem.53.10.2470-2475.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineke W., Knackmuss H. J. Microbial degradation of haloaromatics. Annu Rev Microbiol. 1988;42:263–287. doi: 10.1146/annurev.mi.42.100188.001403. [DOI] [PubMed] [Google Scholar]
- Remberger M., Allard A. S., Neilson A. H. Biotransformations of chloroguaiacols, chlorocatechols, and chloroveratroles in sediments. Appl Environ Microbiol. 1986 Mar;51(3):552–558. doi: 10.1128/aem.51.3.552-558.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remberger M., Hynning P. A., Neilson A. H. Chlorinated benzo-1,2-quinones: an example of chemical transformation of toxicants during tests with aquatic organisms. Ecotoxicol Environ Saf. 1991 Dec;22(3):320–336. doi: 10.1016/0147-6513(91)90082-z. [DOI] [PubMed] [Google Scholar]
- Shields M. S., Hooper S. W., Sayler G. S. Plasmid-mediated mineralization of 4-chlorobiphenyl. J Bacteriol. 1985 Sep;163(3):882–889. doi: 10.1128/jb.163.3.882-889.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


