Abstract
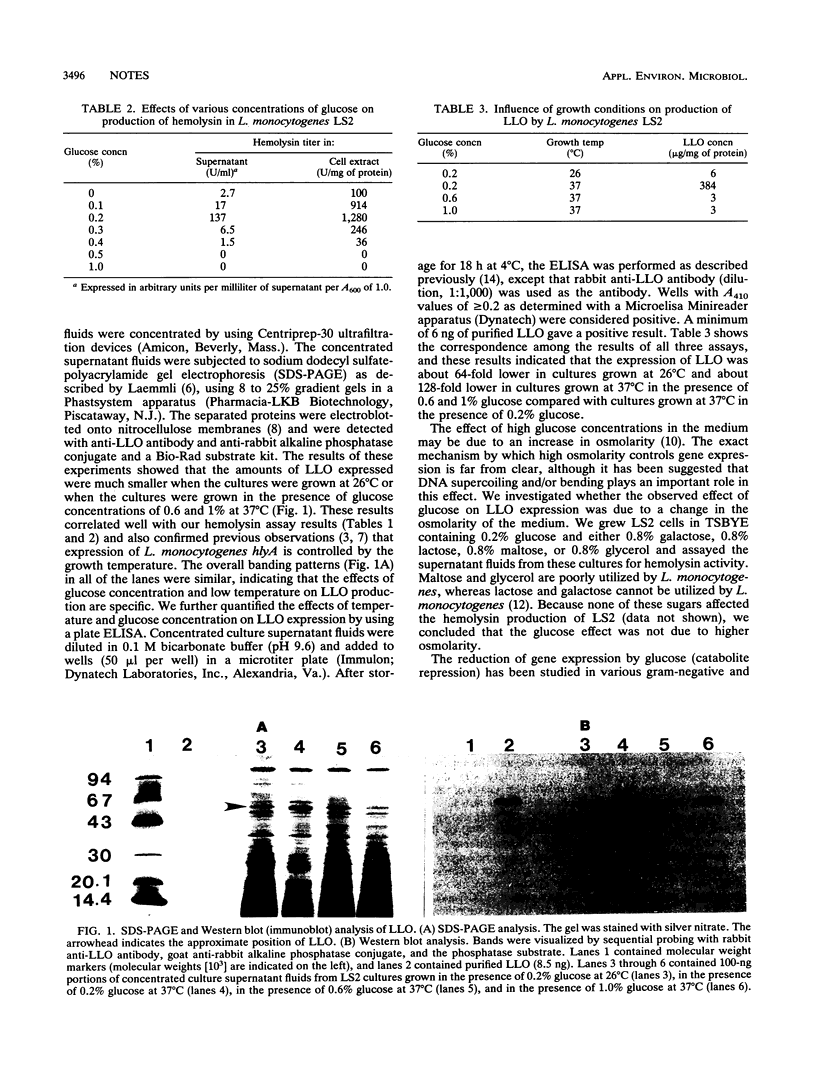
Expression of listeriolysin O of Listeria monocytogenes as a function of different growth conditions was studied by performing a direct hemolysin assay, immunoblotting experiments, and an enzyme-linked immunosorbent assay. Expression of listeriolysin O was reduced at a lower growth temperatures (26 degrees C) and at higher glucose concentrations (> or = 0.3%) in the growth media. The effect of glucose appeared to be due to a change in the pH of the growth media.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botsford J. L., Harman J. G. Cyclic AMP in prokaryotes. Microbiol Rev. 1992 Mar;56(1):100–122. doi: 10.1128/mr.56.1.100-122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Datta A. R., Wentz B. A., Russell J. Cloning of the listeriolysin O gene and development of specific gene probes for Listeria monocytogenes. Appl Environ Microbiol. 1990 Dec;56(12):3874–3877. doi: 10.1128/aem.56.12.3874-3877.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber J. M., Peterkin P. I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991 Sep;55(3):476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leimeister-Wächter M., Domann E., Chakraborty T. The expression of virulence genes in Listeria monocytogenes is thermoregulated. J Bacteriol. 1992 Feb;174(3):947–952. doi: 10.1128/jb.174.3.947-952.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Mekalanos J. J. Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol. 1992 Jan;174(1):1–7. doi: 10.1128/jb.174.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premaratne R. J., Lin W. J., Johnson E. A. Development of an improved chemically defined minimal medium for Listeria monocytogenes. Appl Environ Microbiol. 1991 Oct;57(10):3046–3048. doi: 10.1128/aem.57.10.3046-3048.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regassa L. B., Novick R. P., Betley M. J. Glucose and nonmaintained pH decrease expression of the accessory gene regulator (agr) in Staphylococcus aureus. Infect Immun. 1992 Aug;60(8):3381–3388. doi: 10.1128/iai.60.8.3381-3388.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz A. J., McCardell B. A. DNA homology and immunological cross-reactivity between Aeromonas hydrophila cytotonic toxin and cholera toxin. J Clin Microbiol. 1988 Jan;26(1):57–61. doi: 10.1128/jcm.26.1.57-61.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun A. N., Camilli A., Portnoy D. A. Isolation of Listeria monocytogenes small-plaque mutants defective for intracellular growth and cell-to-cell spread. Infect Immun. 1990 Nov;58(11):3770–3778. doi: 10.1128/iai.58.11.3770-3778.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]