Abstract
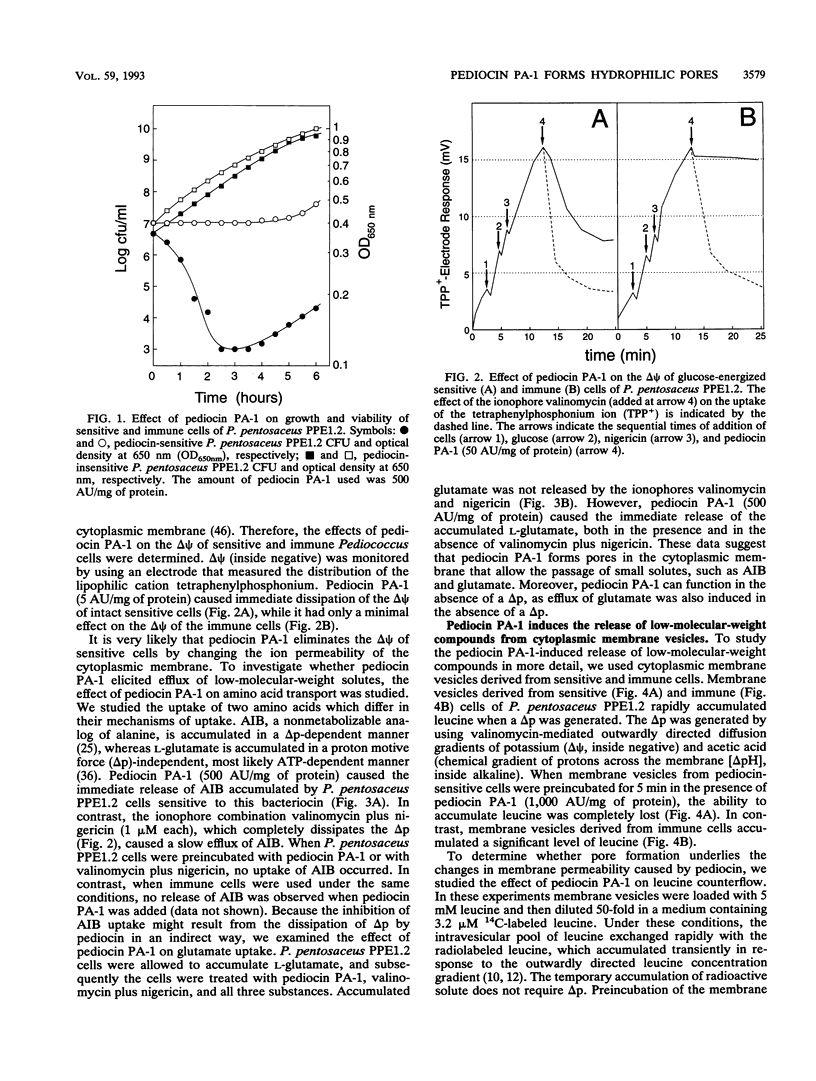
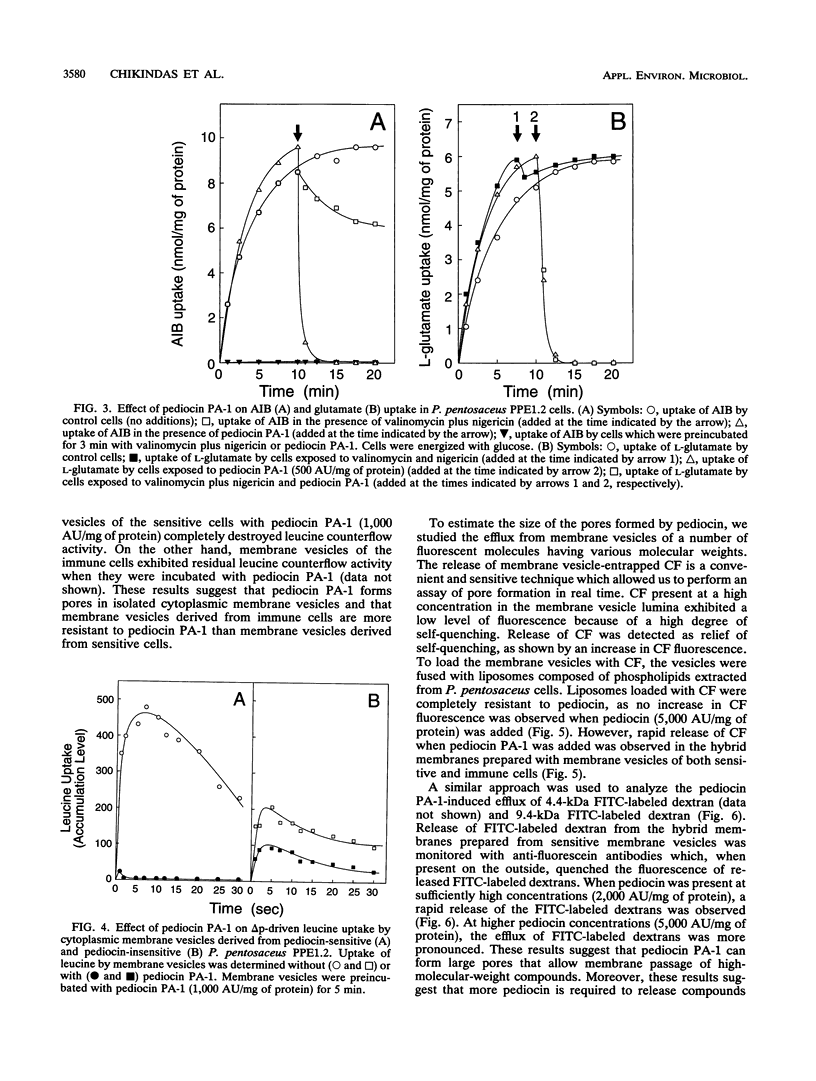
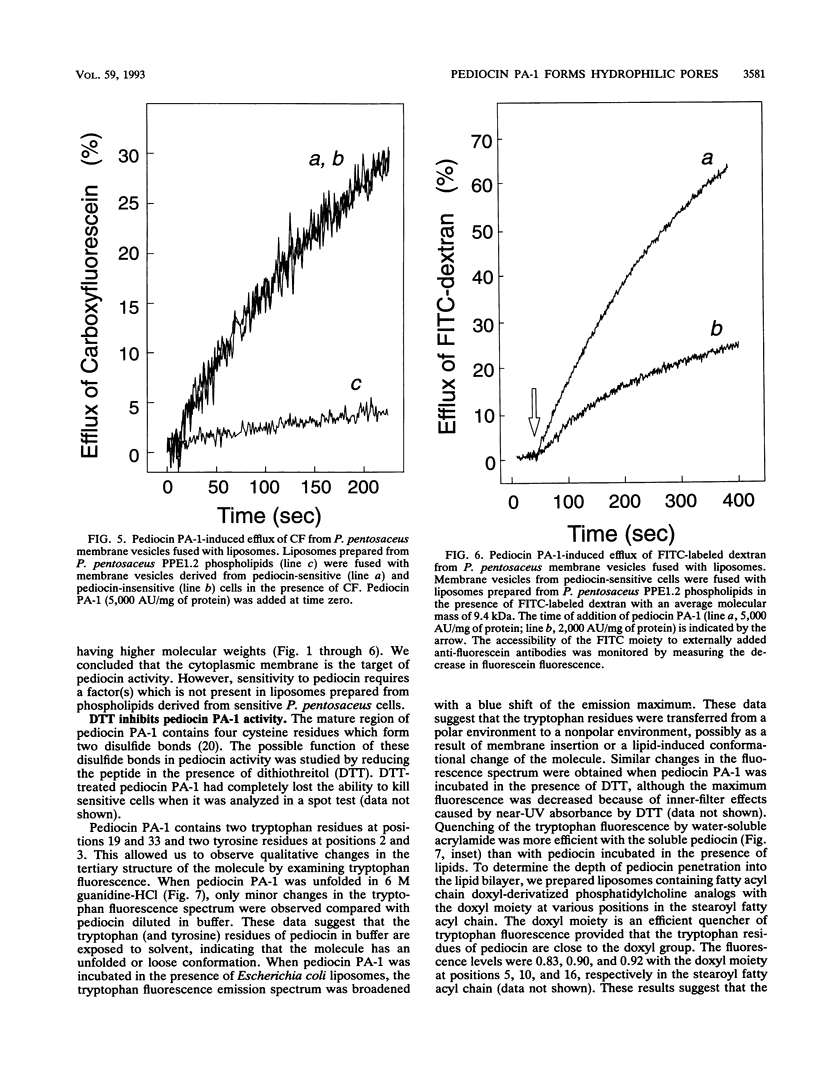
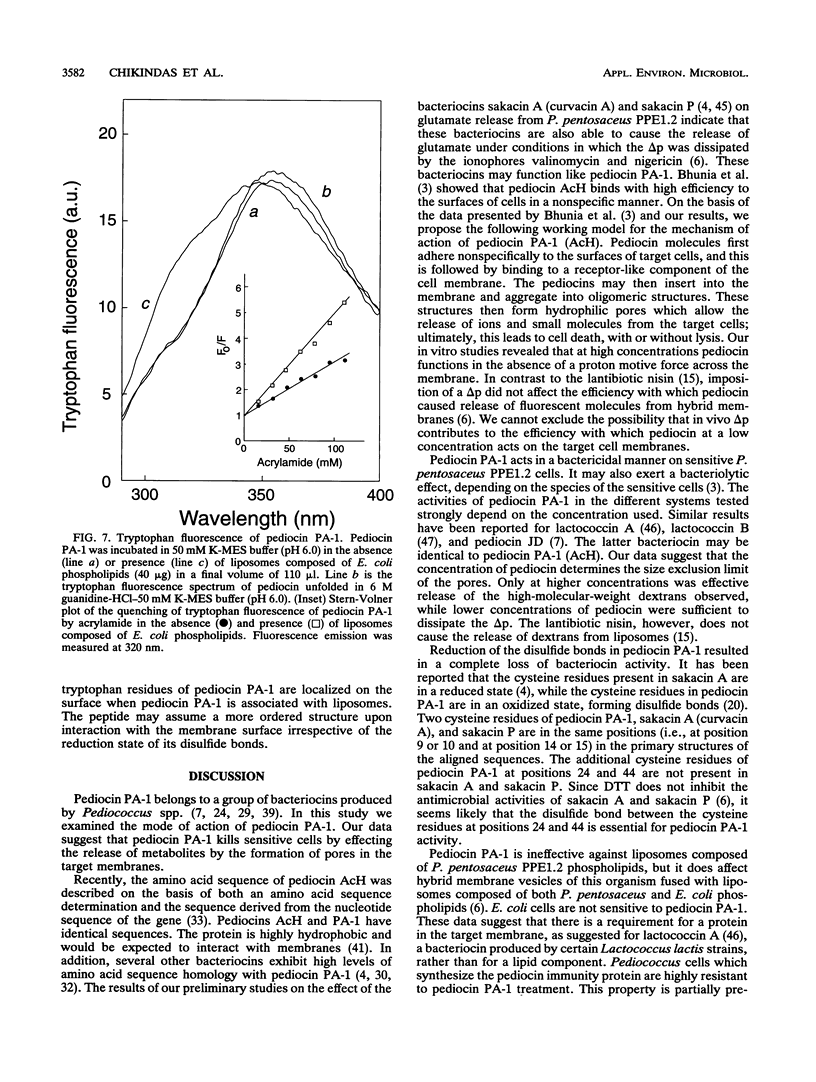
Pediocin PA-1 is a bacteriocin which is produced by Pediococcus acidilactici PAC1.0. We demonstrate that pediocin PA-1 kills sensitive Pediococcus cells and acts on the cytoplasmic membrane. In contrast to its lack of impact on immune cells, pediocin PA-1 dissipates the transmembrane electrical potential and inhibits amino acid transport in sensitive cells. Pediocin interferes with the uptake of amino acids by cytoplasmic membrane vesicles derived from sensitive cells, while it is less effective with membranes derived from immune cells. In liposomes fused with membrane vesicles derived from both sensitive and immune cells, pediocin PA-1 elicits an efflux of small ions and, at higher concentrations, an efflux of molecules having molecular weights of up to 9,400. Our data suggest that pediocin PA-1 functions in a voltage-independent manner but requires a specific protein in the target membrane.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhunia A. K., Johnson M. C., Ray B. Purification, characterization and antimicrobial spectrum of a bacteriocin produced by Pediococcus acidilactici. J Appl Bacteriol. 1988 Oct;65(4):261–268. doi: 10.1111/j.1365-2672.1988.tb01893.x. [DOI] [PubMed] [Google Scholar]
- Bruno M. E., Kaiser A., Montville T. J. Depletion of proton motive force by nisin in Listeria monocytogenes cells. Appl Environ Microbiol. 1992 Jul;58(7):2255–2259. doi: 10.1128/aem.58.7.2255-2259.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen D. P., Hutkins R. W. Collapse of the proton motive force in Listeria monocytogenes caused by a bacteriocin produced by Pediococcus acidilactici. Appl Environ Microbiol. 1992 Oct;58(10):3312–3315. doi: 10.1128/aem.58.10.3312-3315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daeschel M. A., Klaenhammer T. R. Association of a 13.6-Megadalton Plasmid in Pediococcus pentosaceus with Bacteriocin Activity. Appl Environ Microbiol. 1985 Dec;50(6):1538–1541. doi: 10.1128/aem.50.6.1538-1541.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., Kodde J., de Jong S., Konings W. N. Neutral amino acid transport by membrane vesicles of Streptococcus cremoris is subject to regulation by internal pH. J Bacteriol. 1987 Jun;169(6):2748–2754. doi: 10.1128/jb.169.6.2748-2754.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., Konings W. N. Insertion of lipids and proteins into bacterial membranes by fusion with liposomes. Methods Enzymol. 1993;221:394–408. doi: 10.1016/0076-6879(93)21032-4. [DOI] [PubMed] [Google Scholar]
- Driessen A. J., Zheng T., In't Veld G., Op den Kamp J. A., Konings W. N. Lipid requirement of the branched-chain amino acid transport system of Streptococcus cremoris. Biochemistry. 1988 Feb 9;27(3):865–872. doi: 10.1021/bi00403a005. [DOI] [PubMed] [Google Scholar]
- Fitzsimons A., Duffner F., Curtin D., Brophy G., O'kiely P., O'connell M. Assessment of Pediococcus acidilactici as a Potential Silage Inoculant. Appl Environ Microbiol. 1992 Sep;58(9):3047–3052. doi: 10.1128/aem.58.9.3047-3052.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F. H., Abee T., Konings W. N. Mechanism of action of the peptide antibiotic nisin in liposomes and cytochrome c oxidase-containing proteoliposomes. Appl Environ Microbiol. 1991 Aug;57(8):2164–2170. doi: 10.1128/aem.57.8.2164-2170.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcerá M. J., Elferink M. G., Driessen A. J., Konings W. N. In vitro pore-forming activity of the lantibiotic nisin. Role of protonmotive force and lipid composition. Eur J Biochem. 1993 Mar 1;212(2):417–422. doi: 10.1111/j.1432-1033.1993.tb17677.x. [DOI] [PubMed] [Google Scholar]
- Goessens W. H., Driessen A. J., Wilschut J., van Duin J. A synthetic peptide corresponding to the C-terminal 25 residues of phage MS2 coded lysis protein dissipates the protonmotive force in Escherichia coli membrane vesicles by generating hydrophilic pores. EMBO J. 1988 Mar;7(3):867–873. doi: 10.1002/j.1460-2075.1988.tb02886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C. F., Kunka B. S. Evidence for Plasmid Linkage of Raffinose Utilization and Associated alpha-Galactosidase and Sucrose Hydrolase Activity in Pediococcus pentosaceus. Appl Environ Microbiol. 1986 Jan;51(1):105–109. doi: 10.1128/aem.51.1.105-109.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C. F., Kunka B. S. Plasmid-Associated Bacteriocin Production and Sucrose Fermentation in Pediococcus acidilactici. Appl Environ Microbiol. 1987 Oct;53(10):2534–2538. doi: 10.1128/aem.53.10.2534-2538.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. C., McKay L. L. Plasmid DNA in Strains of Pediococcus cerevisiae and Pediococcus pentosaceus. Appl Environ Microbiol. 1985 Aug;50(2):532–534. doi: 10.1128/aem.50.2.532-534.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson J. T., Chopko A. L., van Wassenaar P. D. Purification and primary structure of pediocin PA-1 produced by Pediococcus acidilactici PAC-1.0. Arch Biochem Biophys. 1992 May 15;295(1):5–12. doi: 10.1016/0003-9861(92)90480-k. [DOI] [PubMed] [Google Scholar]
- Holo H., Nilssen O., Nes I. F. Lactococcin A, a new bacteriocin from Lactococcus lactis subsp. cremoris: isolation and characterization of the protein and its gene. J Bacteriol. 1991 Jun;173(12):3879–3887. doi: 10.1128/jb.173.12.3879-3887.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa Y., Kandrach A., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XXVI. Specificity of phospholipids required for energy transfer reactions. J Biol Chem. 1973 Jan 25;248(2):676–684. [PubMed] [Google Scholar]
- Klaenhammer T. R. Bacteriocins of lactic acid bacteria. Biochimie. 1988 Mar;70(3):337–349. doi: 10.1016/0300-9084(88)90206-4. [DOI] [PubMed] [Google Scholar]
- Konings W. N., Poolman B., Driessen A. J. Bioenergetics and solute transport in lactococci. Crit Rev Microbiol. 1989;16(6):419–476. doi: 10.3109/10408418909104474. [DOI] [PubMed] [Google Scholar]
- Konisky J. Colicins and other bacteriocins with established modes of action. Annu Rev Microbiol. 1982;36:125–144. doi: 10.1146/annurev.mi.36.100182.001013. [DOI] [PubMed] [Google Scholar]
- Lewus C. B., Kaiser A., Montville T. J. Inhibition of food-borne bacterial pathogens by bacteriocins from lactic acid bacteria isolated from meat. Appl Environ Microbiol. 1991 Jun;57(6):1683–1688. doi: 10.1128/aem.57.6.1683-1688.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchansky J. B., Glass K. A., Harsono K. D., Degnan A. J., Faith N. G., Cauvin B., Baccus-Taylor G., Arihara K., Bater B., Maurer A. J. Genomic analysis of Pediococcus starter cultures used to control Listeria monocytogenes in turkey summer sausage. Appl Environ Microbiol. 1992 Sep;58(9):3053–3059. doi: 10.1128/aem.58.9.3053-3059.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marugg J. D., Gonzalez C. F., Kunka B. S., Ledeboer A. M., Pucci M. J., Toonen M. Y., Walker S. A., Zoetmulder L. C., Vandenbergh P. A. Cloning, expression, and nucleotide sequence of genes involved in production of pediocin PA-1, and bacteriocin from Pediococcus acidilactici PAC1.0. Appl Environ Microbiol. 1992 Aug;58(8):2360–2367. doi: 10.1128/aem.58.8.2360-2367.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motlagh A. M., Bhunia A. K., Szostek F., Hansen T. R., Johnson M. C., Ray B. Nucleotide and amino acid sequence of pap-gene (pediocin AcH production) in Pediococcus acidilactici H. Lett Appl Microbiol. 1992 Aug;15(2):45–48. doi: 10.1111/j.1472-765x.1992.tb00721.x. [DOI] [PubMed] [Google Scholar]
- Nielsen J. W., Dickson J. S., Crouse J. D. Use of a bacteriocin produced by Pediococcus acidilactici to inhibit Listeria monocytogenes associated with fresh meat. Appl Environ Microbiol. 1990 Jul;56(7):2142–2145. doi: 10.1128/aem.56.7.2142-2145.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto Lozano J. C., Meyer J. N., Sletten K., Peláz C., Nes I. F. Purification and amino acid sequence of a bacteriocin produced by Pediococcus acidilactici. J Gen Microbiol. 1992 Sep;138(9):1985–1990. doi: 10.1099/00221287-138-9-1985. [DOI] [PubMed] [Google Scholar]
- PEDERSON C. S. The genus Pediococcus. Bacteriol Rev. 1949 Dec;13(4):225–232. doi: 10.1128/br.13.4.225-232.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Smid E. J., Veldkamp H., Konings W. N. Bioenergetic consequences of lactose starvation for continuously cultured Streptococcus cremoris. J Bacteriol. 1987 Apr;169(4):1460–1468. doi: 10.1128/jb.169.4.1460-1468.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucci M. J., Vedamuthu E. R., Kunka B. S., Vandenbergh P. A. Inhibition of Listeria monocytogenes by using bacteriocin PA-1 produced by Pediococcus acidilactici PAC 1.0. Appl Environ Microbiol. 1988 Oct;54(10):2349–2353. doi: 10.1128/aem.54.10.2349-2353.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhr E., Sahl H. G. Mode of action of the peptide antibiotic nisin and influence on the membrane potential of whole cells and on cytoplasmic and artificial membrane vesicles. Antimicrob Agents Chemother. 1985 May;27(5):841–845. doi: 10.1128/aac.27.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer M., Chang C. H., Stevens F. J. The functions of tryptophan residues in membrane proteins. Protein Eng. 1992 Apr;5(3):213–214. doi: 10.1093/protein/5.3.213. [DOI] [PubMed] [Google Scholar]
- Schlech W. F., 3rd, Lavigne P. M., Bortolussi R. A., Allen A. C., Haldane E. V., Wort A. J., Hightower A. W., Johnson S. E., King S. H., Nicholls E. S. Epidemic listeriosis--evidence for transmission by food. N Engl J Med. 1983 Jan 27;308(4):203–206. doi: 10.1056/NEJM198301273080407. [DOI] [PubMed] [Google Scholar]
- Shinbo T., Kamo N., Kurihara K., Kobatake Y. A PVC-based electrode sensitive to DDA+ as a device for monitoring the membrane potential in biological systems. Arch Biochem Biophys. 1978 Apr 30;187(2):414–422. doi: 10.1016/0003-9861(78)90052-8. [DOI] [PubMed] [Google Scholar]
- Venema K., Abee T., Haandrikman A. J., Leenhouts K. J., Kok J., Konings W. N., Venema G. Mode of Action of Lactococcin B, a Thiol-Activated Bacteriocin from Lactococcus lactis. Appl Environ Microbiol. 1993 Apr;59(4):1041–1048. doi: 10.1128/aem.59.4.1041-1048.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef A. E., Luchansky J. B., Degnan A. J., Doyle M. P. Behavior of Listeria monocytogenes in wiener exudates in the presence of Pediococcus acidilactici H or pediocin AcH during storage at 4 or 25 degrees C. Appl Environ Microbiol. 1991 May;57(5):1461–1467. doi: 10.1128/aem.57.5.1461-1467.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajdel J. K., Ceglowski P., Dobrazański W. T. Mechanism of action of lactostrepcin 5, a bacteriocin produced by Streptococcus cremoris 202. Appl Environ Microbiol. 1985 Apr;49(4):969–974. doi: 10.1128/aem.49.4.969-974.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Belkum M. J., Kok J., Venema G., Holo H., Nes I. F., Konings W. N., Abee T. The bacteriocin lactococcin A specifically increases permeability of lactococcal cytoplasmic membranes in a voltage-independent, protein-mediated manner. J Bacteriol. 1991 Dec;173(24):7934–7941. doi: 10.1128/jb.173.24.7934-7941.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


