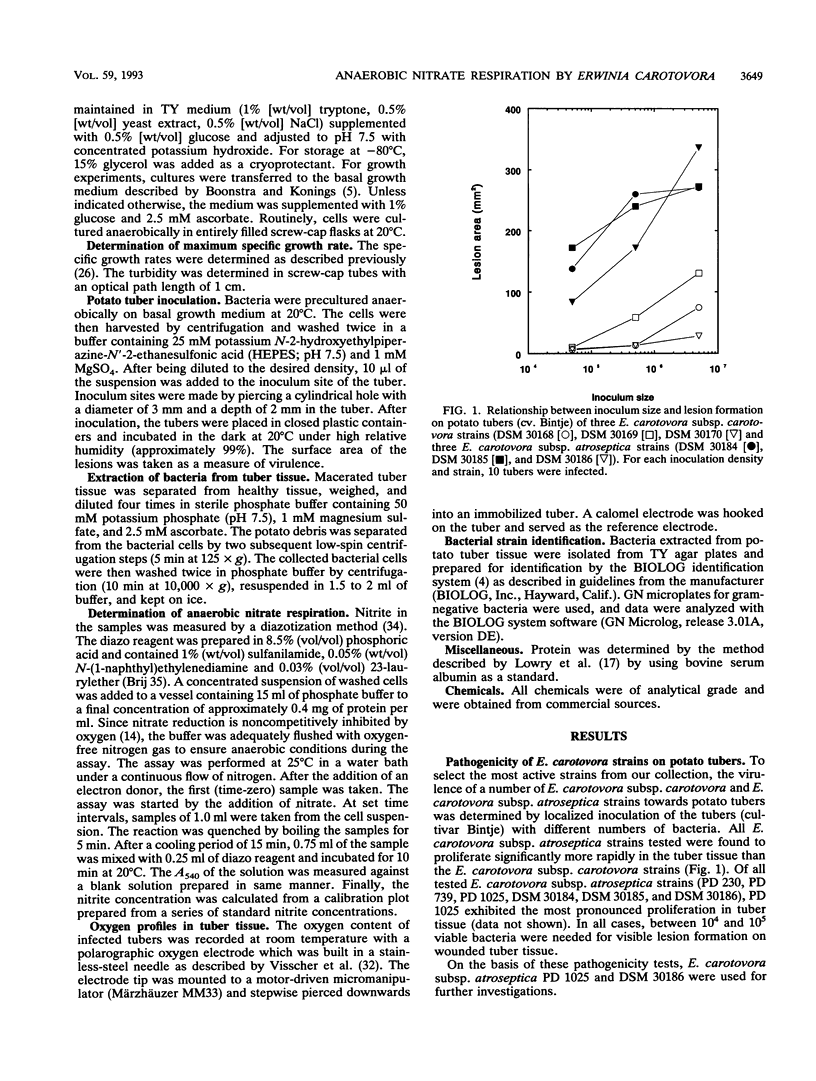
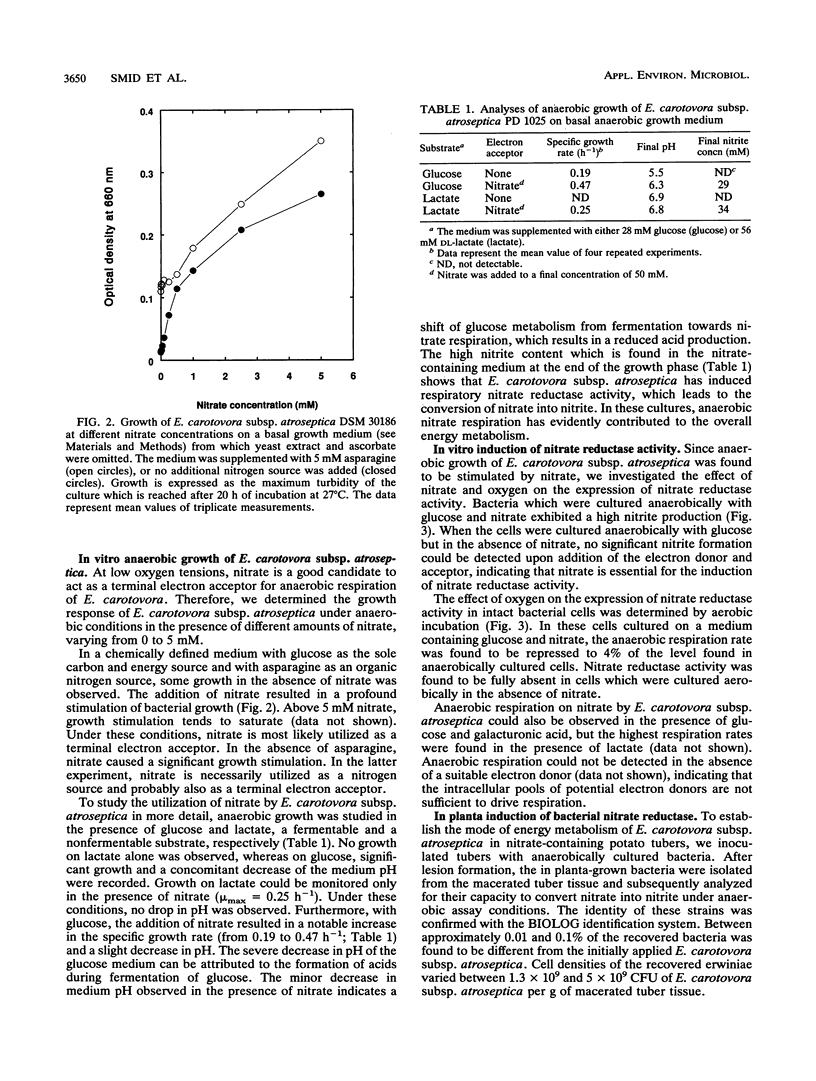
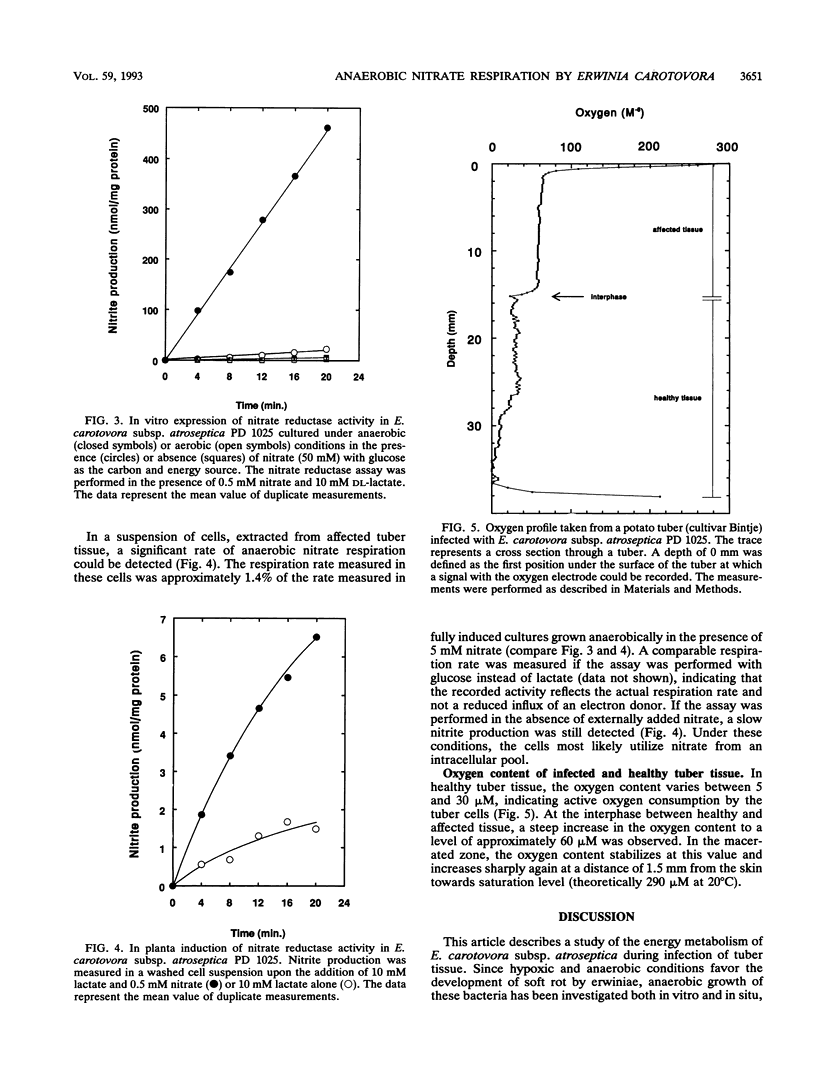
Abstract
The in planta induction of anaerobic nitrate respiration by Erwinia carotovora subsp. atroseptica in relation to the in situ oxygen status in soft rotting potato tubers has been investigated. In vitro experiments have shown that nitrate was required for the induction of respiratory nitrate reductase activity in E. carotovora. In addition, oxygen was found to repress this activity. Expression of respiratory nitrate reductase was found in E. carotovora cells extracted from soft rotting potato tuber tissue. However, the rate of nitrite production in these cells was approximately 70-fold lower than the rate recorded in fully induced anaerobic cultures. Oxygen measurements in soft rotting potato tubers indicated that the invading bacteria encounter the lowest oxygen concentration at the interphase between healthy and macerated tissue. Consequently, growth of bacteria present in this specific zone will be stimulated by nitrate which is present in sufficient amounts in tuber tissue. A high nitrate content of the tuber will most likely facilitate the proliferation of E. carotovora in the tuber tissue.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINSON D. E., MCNALL E. G. Nitrate reduction. I. Growth of Escherichia coli with nitrate as sole source of nitrogen. J Bacteriol. 1956 Aug;72(2):226–229. doi: 10.1128/jb.72.2.226-229.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. R. Sleuthing out bacterial identities. Nature. 1989 May 11;339(6220):157–158. doi: 10.1038/339157a0. [DOI] [PubMed] [Google Scholar]
- Boonstra J., Konings W. N. Generation of an electrochemical proton gradient by nitrate respiration in membrane vesicles from anaerobically grown Escherichia coli. Eur J Biochem. 1977 Sep;78(2):361–368. doi: 10.1111/j.1432-1033.1977.tb11748.x. [DOI] [PubMed] [Google Scholar]
- Butler W., Cook L., Vayda M. E. Hypoxic stress inhibits multiple aspects of the potato tuber wound response. Plant Physiol. 1990 May;93(1):264–270. doi: 10.1104/pp.93.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippaux M., Pichinoty F. Les nitrate-réductases bactériennes. V. Induction de la biosynthèse de l'enzyme A par l'azoture. Arch Mikrobiol. 1970;71(4):361–366. [PubMed] [Google Scholar]
- Forget P. Effect of growth conditions on the synthesis of nitrate reductase components in chlorate resistant mutants of Escherichia coli K 12. Biochem Biophys Res Commun. 1979 Jul 27;89(2):659–663. doi: 10.1016/0006-291x(79)90680-6. [DOI] [PubMed] [Google Scholar]
- Hinton J. C., Sidebotham J. M., Hyman L. J., Pérombelon M. C., Salmond G. P. Isolation and characterisation of transposon-induced mutants of Erwinia carotovora subsp. atroseptica exhibiting reduced virulence. Mol Gen Genet. 1989 May;217(1):141–148. doi: 10.1007/BF00330953. [DOI] [PubMed] [Google Scholar]
- John P. Aerobic and anaerobic bacterial respiration monitored by electrodes. J Gen Microbiol. 1977 Jan;98(1):231–238. doi: 10.1099/00221287-98-1-231. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McNALL E. G., ATKINSON D. E. Nitrate reduction. II. Utilization of possible intermediates as nitrogen sources and as electron acceptors. J Bacteriol. 1957 Jul;74(1):60–66. doi: 10.1128/jb.74.1.60-66.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Herrera J., Salas-Vargas I. Regulation of nitrate reductase at the transcriptional and translational levels in Escherichia coli. Biochim Biophys Acta. 1976 Apr 2;425(4):492–501. doi: 10.1016/0005-2787(76)90013-7. [DOI] [PubMed] [Google Scholar]
- Smid E. J., Plapp R., Konings W. N. Peptide uptake is essential for growth of Lactococcus lactis on the milk protein casein. J Bacteriol. 1989 Nov;171(11):6135–6140. doi: 10.1128/jb.171.11.6135-6140.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuyumu S. Inducer of pectic acid lyase in Erwinia carotovora. Nature. 1977 Sep 15;269(5625):237–238. doi: 10.1038/269237a0. [DOI] [PubMed] [Google Scholar]
- Vayda M. E., Schaeffer H. J. Hypoxic stress inhibits the appearance of wound-response proteins in potato tubers. Plant Physiol. 1988 Nov;88(3):805–809. doi: 10.1104/pp.88.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser R. G., Hellingwerf K. J., Konings W. N. The protein composition of the cytoplasmic membrane of aerobically and anaerobically grown Escherichia coli. J Bioenerg Biomembr. 1984 Aug;16(4):295–307. doi: 10.1007/BF00744282. [DOI] [PubMed] [Google Scholar]
- Zink R. T., Engwall J. K., McEvoy J. L., Chatterjee A. K. recA is required in the induction of pectin lyase and carotovoricin in Erwinia carotovora subsp. carotovora. J Bacteriol. 1985 Oct;164(1):390–396. doi: 10.1128/jb.164.1.390-396.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


