Abstract
Five marine cyanophages propagated on Synechococcus sp. strain WH7803 were isolated from three different oceanographic provinces during the months of August and September 1992: coastal water from the Sargasso Sea, Bermuda; Woods Hole harbor, Woods Hole, Mass.; and coastal water from the English Channel, off Plymouth Sound, United Kingdom. The five cyanophage isolates were found to belong to two families, Myoviridae and Styloviridae, on the basis of their morphology observed in the transmission electron microscope. DNA purified from each of the cyanophage isolates was restricted with a selection of restriction endonucleases, and three distinguishably different patterns were observed. DNA isolated from Myoviridae isolates from Bermuda and the English Channel had highly related restriction patterns, as did DNA isolated from Styloviridae isolates from Bermuda and the English Channel. DNA isolated from the Myoviridae isolate from Woods Hole had a unique restriction pattern. The genome size for each of the Myoviridae isolates was ca. 80 to 85 kb, and it was ca. 90 to 100 kb for each of the Styloviridae isolates. Southern blotting analysis revealed that there was a limited degree of homology among all cyanophage DNAs probed, but clear differences were observed between cyanophage DNA from the Myoviridae and that from the Styloviridae isolates. Polypeptide analysis revealed a clear difference between Myoviridae and Styloviridae polypeptide profiles, although the major, presumably structural, protein in each case was ca. 53 to 54 kDa.
Full text
PDF




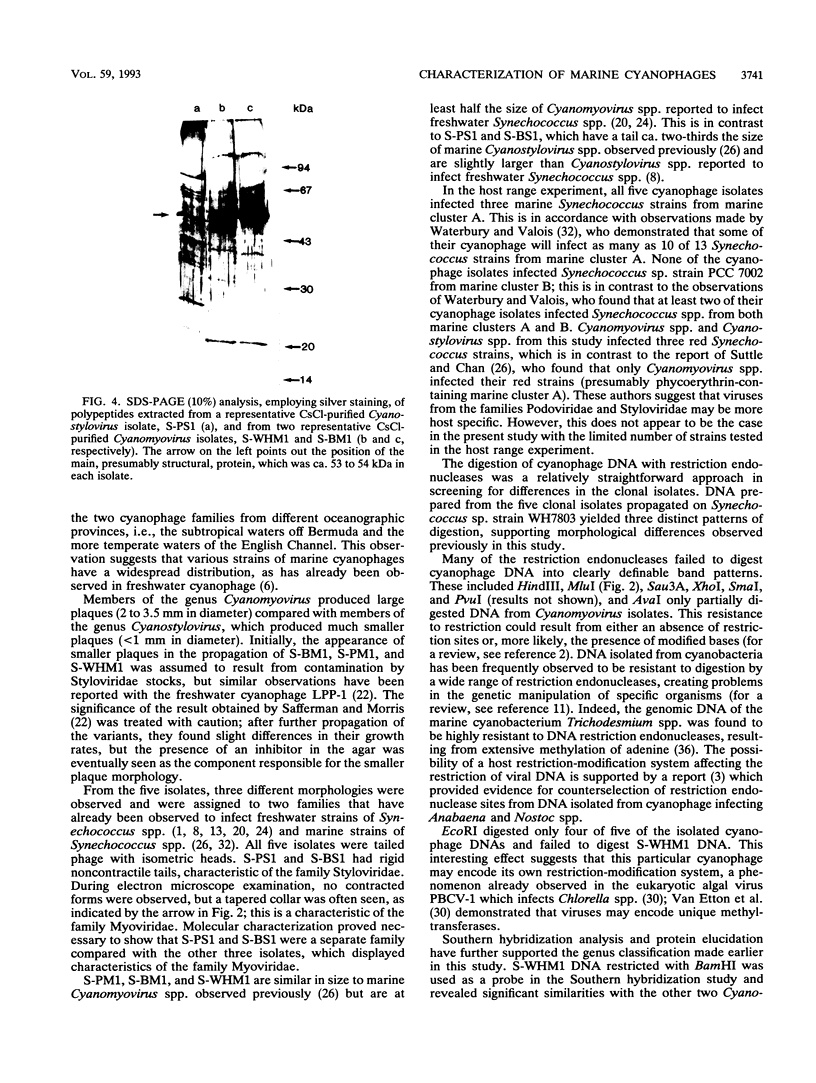


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adolph K. W., Haselkorn R. Photosynthesis and the development of blue-green algal virus N-1. Virology. 1972 Feb;47(2):370–374. doi: 10.1016/0042-6822(72)90272-3. [DOI] [PubMed] [Google Scholar]
- Arber W., Linn S. DNA modification and restriction. Annu Rev Biochem. 1969;38:467–500. doi: 10.1146/annurev.bi.38.070169.002343. [DOI] [PubMed] [Google Scholar]
- Bancroft I., Smith R. J. An analysis of restriction endonuclease sites in cyanophages infecting the heterocystous cyanobacteria Anabaena and Nostoc. J Gen Virol. 1988 Mar;69(Pt 3):739–743. doi: 10.1099/0022-1317-69-3-739. [DOI] [PubMed] [Google Scholar]
- Bergh O., Børsheim K. Y., Bratbak G., Heldal M. High abundance of viruses found in aquatic environments. Nature. 1989 Aug 10;340(6233):467–468. doi: 10.1038/340467a0. [DOI] [PubMed] [Google Scholar]
- Bratbak G., Heldal M., Norland S., Thingstad T. F. Viruses as partners in spring bloom microbial trophodynamics. Appl Environ Microbiol. 1990 May;56(5):1400–1405. doi: 10.1128/aem.56.5.1400-1405.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fox J. A., Booth S. J., Martin E. L. Cyanophage SM-2: a new blue-green algal virus. Virology. 1976 Sep;73(2):557–560. doi: 10.1016/0042-6822(76)90420-7. [DOI] [PubMed] [Google Scholar]
- Khudyakov I. Y., Kirnos M. D., Alexandrushkina N. I., Vanyushin B. F. Cyanophage S-2L contains DNA with 2,6-diaminopurine substituted for adenine. Virology. 1978 Jul 1;88(1):8–18. doi: 10.1016/0042-6822(78)90104-6. [DOI] [PubMed] [Google Scholar]
- Padan E., Shilo M. Cyanophages-viruses attacking blue-green algae. Bacteriol Rev. 1973 Sep;37(3):343–370. doi: 10.1128/br.37.3.343-370.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAFFERMAN R. S., MORRIS M. E. Algal virus: isolation. Science. 1963 May 10;140(3567):679–680. doi: 10.1126/science.140.3567.679. [DOI] [PubMed] [Google Scholar]
- SAFFERMAN R. S., MORRIS M. E. GROWTH CHARACTERISTICS OF THE BLUE-GREEN ALGAL VIRUS LPP-1. J Bacteriol. 1964 Sep;88:771–775. doi: 10.1128/jb.88.3.771-775.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safferman R. S., Cannon R. E., Desjardins P. R., Gromov B. V., Haselkorn R., Sherman L. A., Shilo M. Classification and nomenclature of viruses of cyanobacteria. Intervirology. 1983;19(2):61–66. doi: 10.1159/000149339. [DOI] [PubMed] [Google Scholar]
- Safferman R. S., Diener T. O., Desjardins P. R., Morris M. E. Isolation and characterization of AS-1, a phycovirus infecting the blue-green algae, Anacystis nidulans and Synechococcus cedrorum. Virology. 1972 Jan;47(1):105–113. doi: 10.1016/0042-6822(72)90243-7. [DOI] [PubMed] [Google Scholar]
- Sherman L. A., Connelly M. Isolation and characterization of a cyanophage infecting the unicellular blue-green algae A. nidulans and S. cedrorum. Virology. 1976 Jul 15;72(2):540–544. doi: 10.1016/0042-6822(76)90186-0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Suttle C. A., Chan A. M., Cottrell M. T. Use of ultrafiltration to isolate viruses from seawater which are pathogens of marine phytoplankton. Appl Environ Microbiol. 1991 Mar;57(3):721–726. doi: 10.1128/aem.57.3.721-726.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle C. A., Chen F. Mechanisms and rates of decay of marine viruses in seawater. Appl Environ Microbiol. 1992 Nov;58(11):3721–3729. doi: 10.1128/aem.58.11.3721-3729.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten J. L., Schuster A. M., Girton L., Burbank D. E., Swinton D., Hattman S. DNA methylation of viruses infecting a eukaryotic Chlorella-like green alga. Nucleic Acids Res. 1985 May 24;13(10):3471–3478. doi: 10.1093/nar/13.10.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Wyman M., Gregory R. P., Carr N. G. Novel Role for Phycoerythrin in a Marine Cyanobacterium, Synechococcus Strain DC2. Science. 1985 Nov 15;230(4727):818–820. doi: 10.1126/science.230.4727.818. [DOI] [PubMed] [Google Scholar]
- Zehr J. P., Ohki K., Fujita Y., Landry D. Unique modification of adenine in genomic DNA of the marine cyanobacterium Trichodesmium sp. strain NIBB 1067. J Bacteriol. 1991 Nov;173(21):7059–7062. doi: 10.1128/jb.173.21.7059-7062.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]






