Abstract
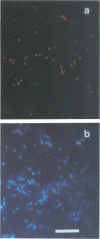
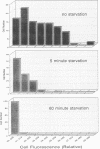
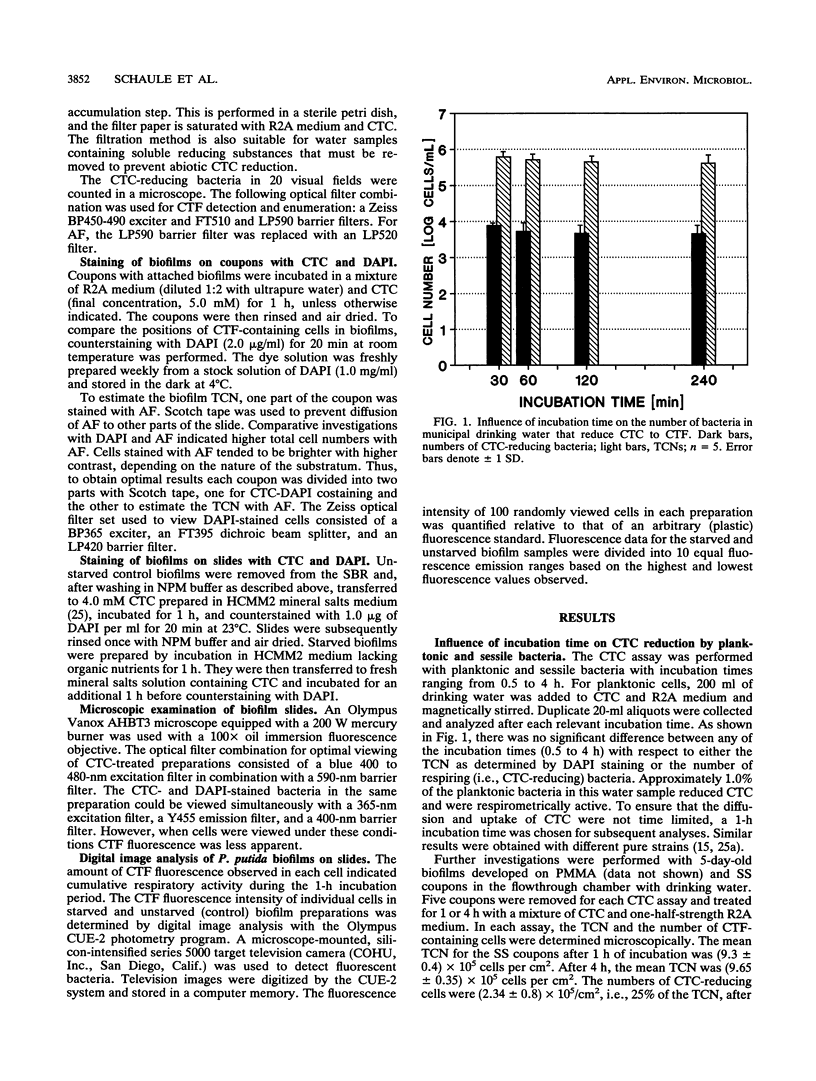
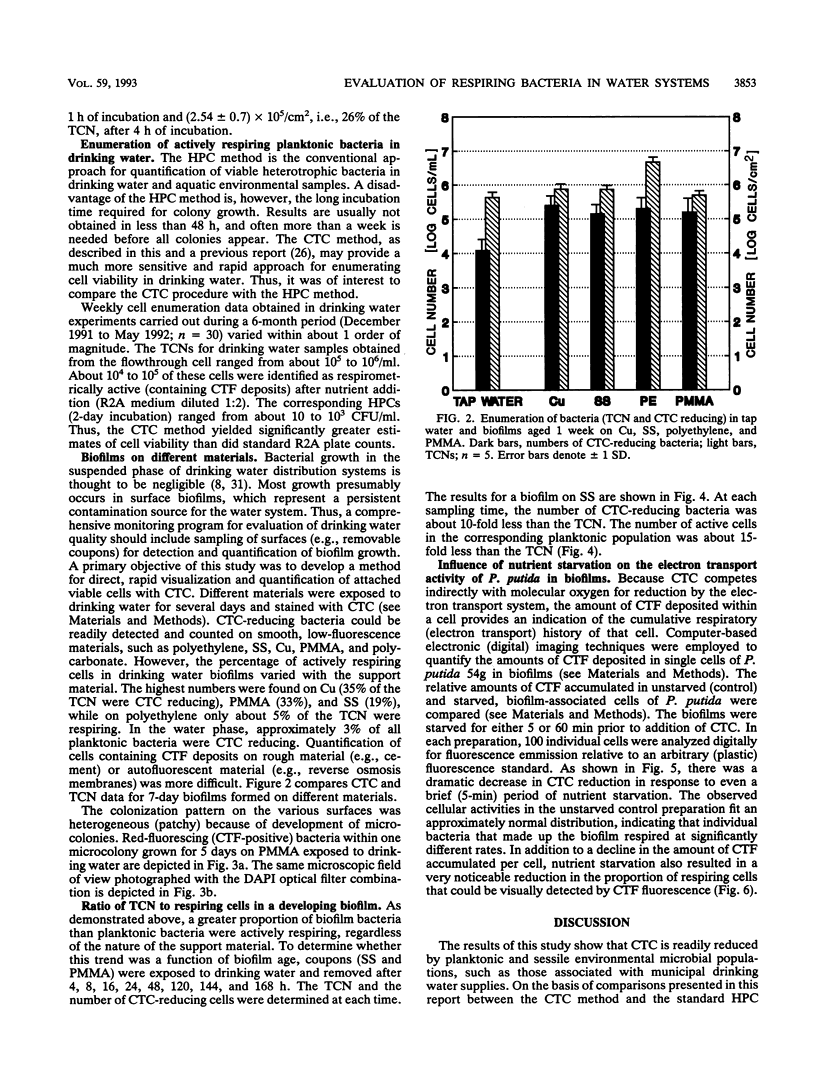
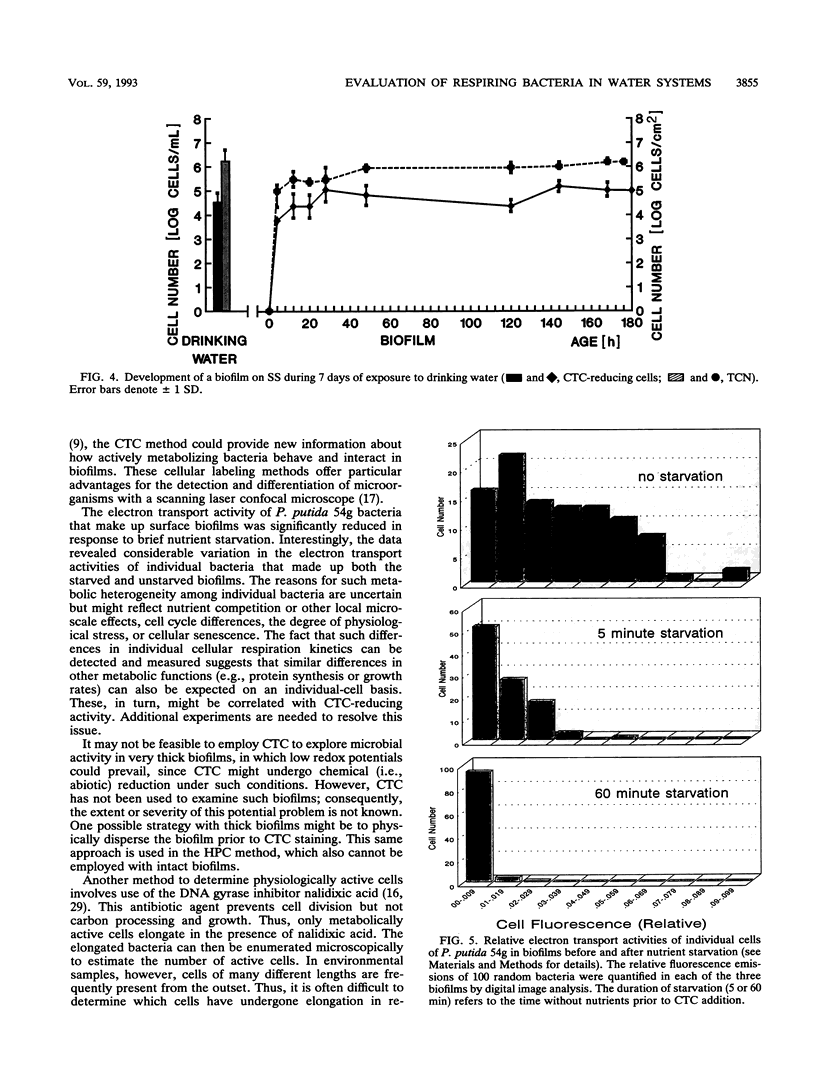
Direct microscopic quantification of respiring (i.e., viable) bacteria was performed for drinking water samples and biofilms grown on different opaque substrata. Water samples or biofilms developed in flowing drinking water were incubated with the vital redox dye 5-cyano-2,3-ditolyl tetrazolium chloride (CTC) and R2A medium. One hour of incubation with 0.5 mM CTC was sufficient to obtain intracellular reduction of CTC to the insoluble fluorescent formazan (CTF) product, which was indicative of cellular respiratory (i.e., electron transport) activity. This result was obtained with both planktonic and biofilm-associated cells. Planktonic bacteria were captured on 0.2-microns-pore-size polycarbonate membrane filters and examined by epifluorescence microscopy. Respiring cells containing CTF deposits were readily detected and quantified as red-fluorescing objects on a dark background. The number of CTC-reducing bacteria was consistently greater than the number of aerobic CFU determined on R2A medium. Approximately 1 to 10% of the total planktonic population (determined by counterstaining with 4,6-diamidino-2-phenylindole) were respirometrically active. The proportion of respiring bacteria in biofilms composed of drinking water microflora was greater, ranging from about 5 to 35%, depending on the substratum. Respiring cells were distributed more or less evenly in biofilms, as demonstrated by counterstaining with 4,6-diamidino-2-phenylindole. The amount of CTF deposited in single cells of Pseudomonas putida that formed monospecies biofilms was quantified by digital image analysis and used to indicate cumulative respiratory activity. These data indicated significant cell-to-cell variation in respiratory activity and reduced electron transport following a brief period of nutrient starvation.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman F. P. On the oxygen-sensitivity of various tetrazolium salts. Histochemie. 1970;22(3):256–261. doi: 10.1007/BF00306103. [DOI] [PubMed] [Google Scholar]
- Amann R. I., Stromley J., Devereux R., Key R., Stahl D. A. Molecular and microscopic identification of sulfate-reducing bacteria in multispecies biofilms. Appl Environ Microbiol. 1992 Feb;58(2):614–623. doi: 10.1128/aem.58.2.614-623.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström I., Heinänen A., Salonen K. Comparison of acridine orange, acriflavine, and bisbenzimide stains for enumeration of bacteria in clear and humic waters. Appl Environ Microbiol. 1986 Mar;51(3):664–667. doi: 10.1128/aem.51.3.664-667.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitton G., Koopman B. Tetrazolium reduction-malachite green method for assessing the viability of filamentous bacteria in activated sludge. Appl Environ Microbiol. 1982 Apr;43(4):964–966. doi: 10.1128/aem.43.4.964-966.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bric J. M., Bostock R. M., Silverstone S. E. Rapid in situ assay for indoleacetic Acid production by bacteria immobilized on a nitrocellulose membrane. Appl Environ Microbiol. 1991 Feb;57(2):535–538. doi: 10.1128/aem.57.2.535-538.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dott W., Schoenen D. Qualitative und quantitative Bestimmung von Bakterienpopulationen aus aquatischen Biotopen. 7. Mitteilung: Entwicklung der Aufwuchsflora auf Werkstoffen im Trinkwasser. Zentralbl Bakteriol Mikrobiol Hyg B. 1985 May;180(5-6):436–447. [PubMed] [Google Scholar]
- Kogure K., Simidu U., Taga N. Distribution of viable marine bacteria in neritic seawater around Japan. Can J Microbiol. 1980 Mar;26(3):318–323. doi: 10.1139/m80-052. [DOI] [PubMed] [Google Scholar]
- Lawrence J. R., Korber D. R., Hoyle B. D., Costerton J. W., Caldwell D. E. Optical sectioning of microbial biofilms. J Bacteriol. 1991 Oct;173(20):6558–6567. doi: 10.1128/jb.173.20.6558-6567.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeChevallier M. W., Cawthon C. D., Lee R. G. Inactivation of biofilm bacteria. Appl Environ Microbiol. 1988 Oct;54(10):2492–2499. doi: 10.1128/aem.54.10.2492-2499.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard C., Ponsonnet C., Paget E., Nesme X., Simonet P. Detection and enumeration of bacteria in soil by direct DNA extraction and polymerase chain reaction. Appl Environ Microbiol. 1992 Sep;58(9):2717–2722. doi: 10.1128/aem.58.9.2717-2722.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reasoner D. J., Geldreich E. E. A new medium for the enumeration and subculture of bacteria from potable water. Appl Environ Microbiol. 1985 Jan;49(1):1–7. doi: 10.1128/aem.49.1.1-7.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway H. F., Means E. G., Olson B. H. Iron bacteria in drinking-water distribution systems: elemental analysis of gallionella stalks, using x-ray energy-dispersive microanalysis. Appl Environ Microbiol. 1981 Jan;41(1):288–297. doi: 10.1128/aem.41.1.288-297.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgway H. F., Safarik J., Phipps D., Carl P., Clark D. Identification and catabolic activity of well-derived gasoline-degrading bacteria from a contaminated aquifer. Appl Environ Microbiol. 1990 Nov;56(11):3565–3575. doi: 10.1128/aem.56.11.3565-3575.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez G. G., Phipps D., Ishiguro K., Ridgway H. F. Use of a fluorescent redox probe for direct visualization of actively respiring bacteria. Appl Environ Microbiol. 1992 Jun;58(6):1801–1808. doi: 10.1128/aem.58.6.1801-1808.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A., Pyle B. H., McFeters G. A. Rapid enumeration of viable bacteria by image analysis. J Microbiol Methods. 1989;10:91–101. doi: 10.1016/0167-7012(89)90005-5. [DOI] [PubMed] [Google Scholar]
- Zambon J. J., Huber P. S., Meyer A. E., Slots J., Fornalik M. S., Baier R. E. In situ identification of bacterial species in marine microfouling films by using an immunofluorescence technique. Appl Environ Microbiol. 1984 Dec;48(6):1214–1220. doi: 10.1128/aem.48.6.1214-1220.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]