Abstract
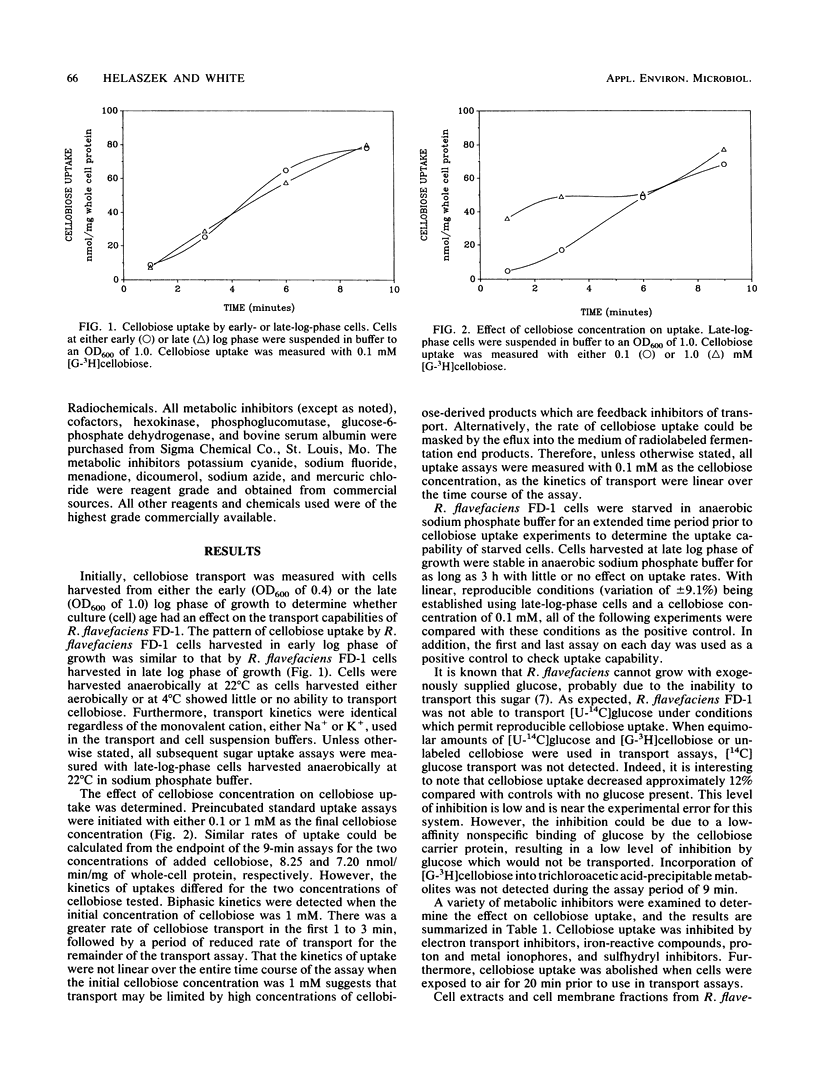
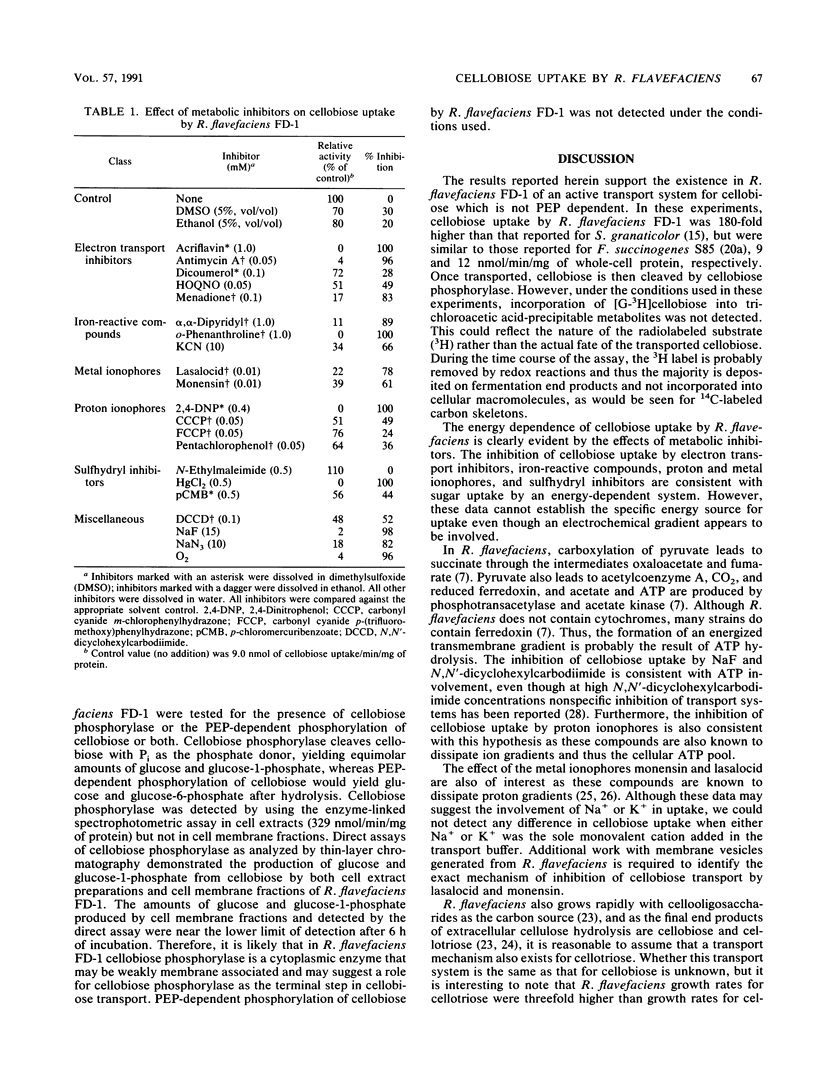
The cellulolytic ruminal bacterium Ruminococcus flavefaciens FD-1 utilizes cellobiose but not glucose as a substrate for growth. Cellobiose uptake by R. flavefaciens FD-1 was measured under anaerobic conditions (N2), using [G-3H]cellobiose. The rate of cellobiose uptake for early- or late-log-phase cellobiose-grown cells was 9 nmol/min per mg of whole-cell protein. Cellobiose uptake was inhibited by electron transport inhibitors, iron-reactive compounds, proton ionophores, sulfhydryl inhibitors, N,N-dicyclohexylcarbodiimide, and NaF, as well as lasalocid and monensin. The results support the existence of an active transport system for cellobiose. Transport of [U-14C]glucose was not detected with this system. Phosphorylation of cellobiose was not by a phosphoenolpyruvate-dependent system. Cellobiose phosphorylase activity was detected by both a coupled spectrophotometric assay and a discontinuous assay. The enzyme was produced constitutively in cellobiose-grown cells at a specific activity of 329 nmol/min per mg of cell-free extract protein.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AYERS W. A. Phosphorolysis and synthesis of cellobiose by cell extracts from Ruminococcus flavefaciens. J Biol Chem. 1959 Nov;234:2819–2822. [PubMed] [Google Scholar]
- AYERS W. A. Phosphorylation of cellobiose and glucose by Ruminococcus flavefaciens. J Bacteriol. 1958 Nov;76(5):515–517. doi: 10.1128/jb.76.5.515-517.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT M. P. Bacterial species of the rumen. Bacteriol Rev. 1959 Sep;23(3):125–153. doi: 10.1128/br.23.3.125-153.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barras F., Chambost J. P., Chippaux M. Cellobiose metabolism in Erwinia: genetic study. Mol Gen Genet. 1984;197(3):486–490. doi: 10.1007/BF00329947. [DOI] [PubMed] [Google Scholar]
- Bernier R., Stutzenberger F. Preferential Utilization of Cellobiose by Thermomonospora curvata. Appl Environ Microbiol. 1987 Aug;53(8):1743–1747. doi: 10.1128/aem.53.8.1743-1747.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P. Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr. 1972 Dec;25(12):1324–1328. doi: 10.1093/ajcn/25.12.1324. [DOI] [PubMed] [Google Scholar]
- Champion K. M., Helaszek C. T., White B. A. Analysis of antibiotic susceptibility and extrachromosomal DNA content of Ruminococcus albus and Ruminococcus flavefaciens. Can J Microbiol. 1988 Oct;34(10):1109–1115. doi: 10.1139/m88-196. [DOI] [PubMed] [Google Scholar]
- Doerner K. C., White B. A. Assessment of the endo-1,4-beta-glucanase components of Ruminococcus flavefaciens FD-1. Appl Environ Microbiol. 1990 Jun;56(6):1844–1850. doi: 10.1128/aem.56.6.1844-1850.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklund C. V., Glass T. L. Glucose uptake by the cellulolytic ruminal anaerobe Bacteroides succinogenes. J Bacteriol. 1987 Feb;169(2):500–506. doi: 10.1128/jb.169.2.500-506.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner R. M., Doerner K. C., White B. A. Purification and characterization of an exo-beta-1,4-glucanase from Ruminococcus flavefaciens FD-1. J Bacteriol. 1987 Oct;169(10):4581–4588. doi: 10.1128/jb.169.10.4581-4588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylemon P. B., Young J. L., Roadcap R. F., Phibbs P. V., Jr Uptake and incorporation of glucose and mannose by whole cells of Bacteroides thetaiotaomicron. Appl Environ Microbiol. 1977 Nov;34(5):488–494. doi: 10.1128/aem.34.5.488-494.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalb V. F., Jr, Bernlohr R. W. A new spectrophotometric assay for protein in cell extracts. Anal Biochem. 1977 Oct;82(2):362–371. doi: 10.1016/0003-2697(77)90173-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martin S. A., Russell J. B. Transport and phosphorylation of disaccharides by the ruminal bacterium Streptococcus bovis. Appl Environ Microbiol. 1987 Oct;53(10):2388–2393. doi: 10.1128/aem.53.10.2388-2393.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T. K., Zeikus J. G. Differential metabolism of cellobiose and glucose by Clostridium thermocellum and Clostridium thermohydrosulfuricum. J Bacteriol. 1982 Jun;150(3):1391–1399. doi: 10.1128/jb.150.3.1391-1399.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen M. A., Hespell R. B., White B. A., Bothast R. J. Inhibitory Effects of Methylcellulose on Cellulose Degradation by Ruminococcus flavefaciens. Appl Environ Microbiol. 1988 Apr;54(4):890–897. doi: 10.1128/aem.54.4.890-897.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B. A proposed mechanism of monensin action in inhibiting ruminal bacterial growth: effects on ion flux and protonmotive force. J Anim Sci. 1987 May;64(5):1519–1525. doi: 10.2527/jas1987.6451519x. [DOI] [PubMed] [Google Scholar]
- Russell J. B. Fermentation of cellodextrins by cellulolytic and noncellulolytic rumen bacteria. Appl Environ Microbiol. 1985 Mar;49(3):572–576. doi: 10.1128/aem.49.3.572-576.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Strobel H. J. Effect of ionophores on ruminal fermentation. Appl Environ Microbiol. 1989 Jan;55(1):1–6. doi: 10.1128/aem.55.1.1-6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A. P., Bragg P. D. Effect of dicyclohexylcarbodiimide on growth and membrane-mediated processes in wild type and heptose-deficient mutants of Escherichia coli K-12. J Bacteriol. 1974 Jul;119(1):129–137. doi: 10.1128/jb.119.1.129-137.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


