Abstract
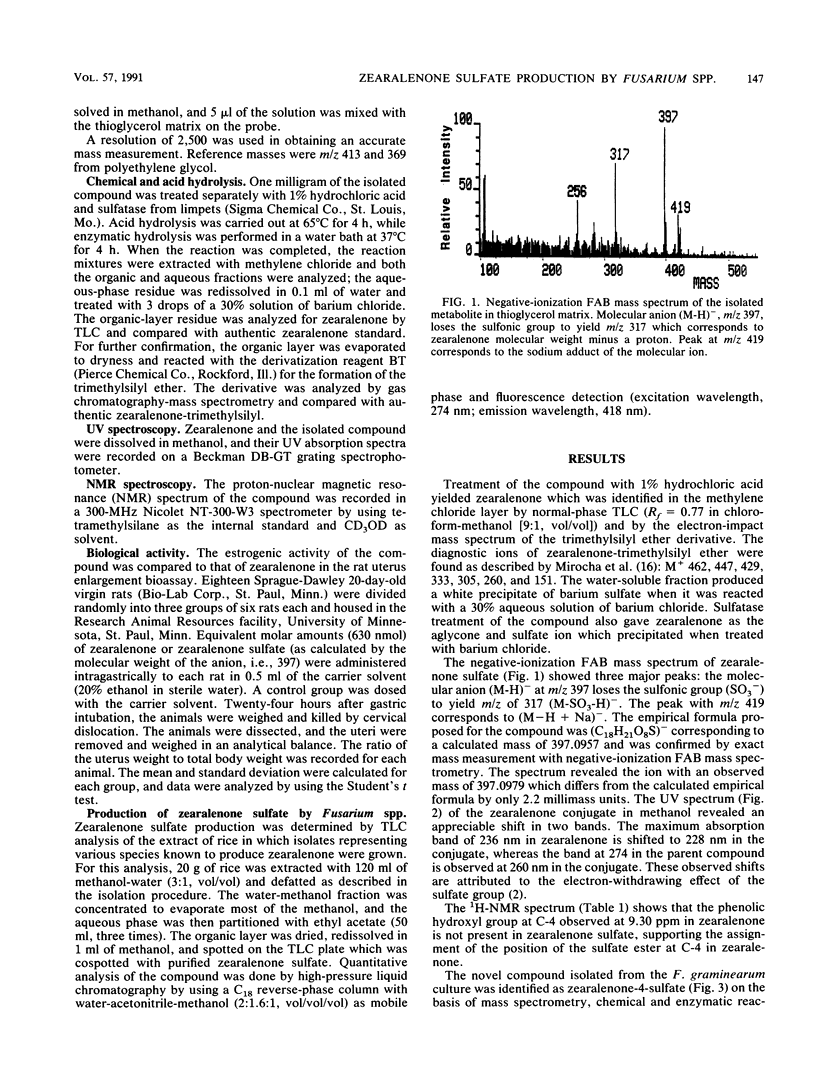
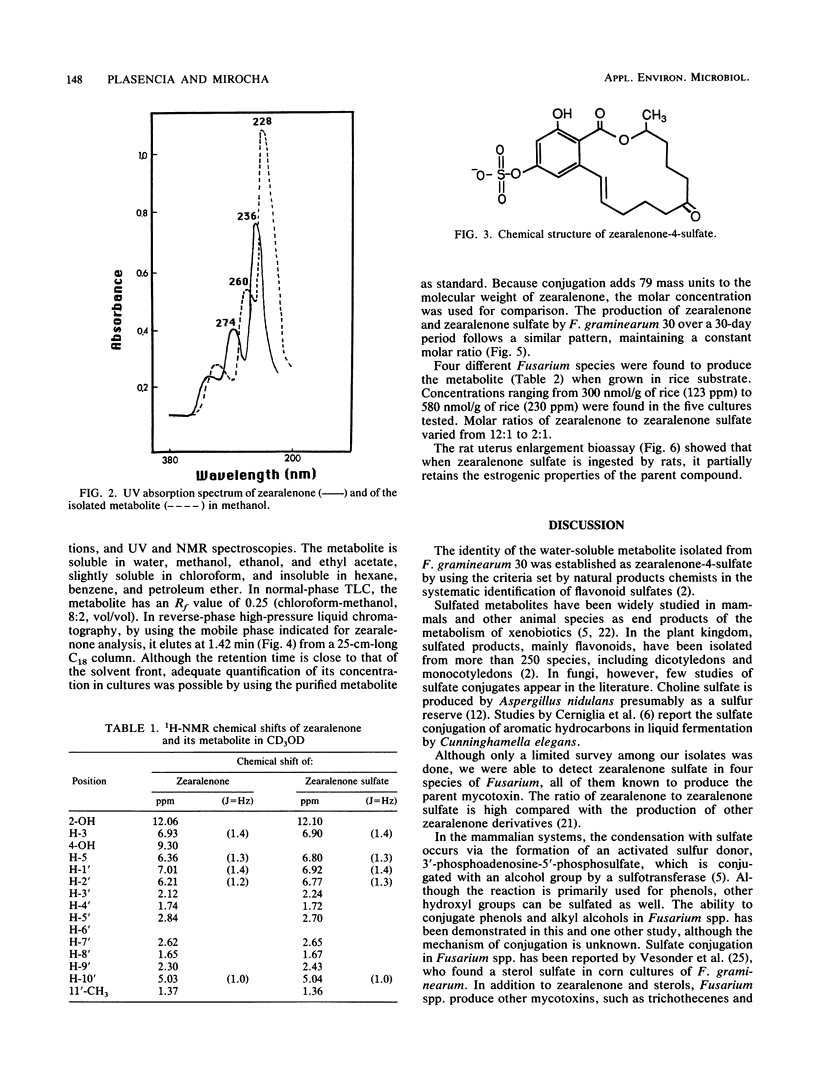
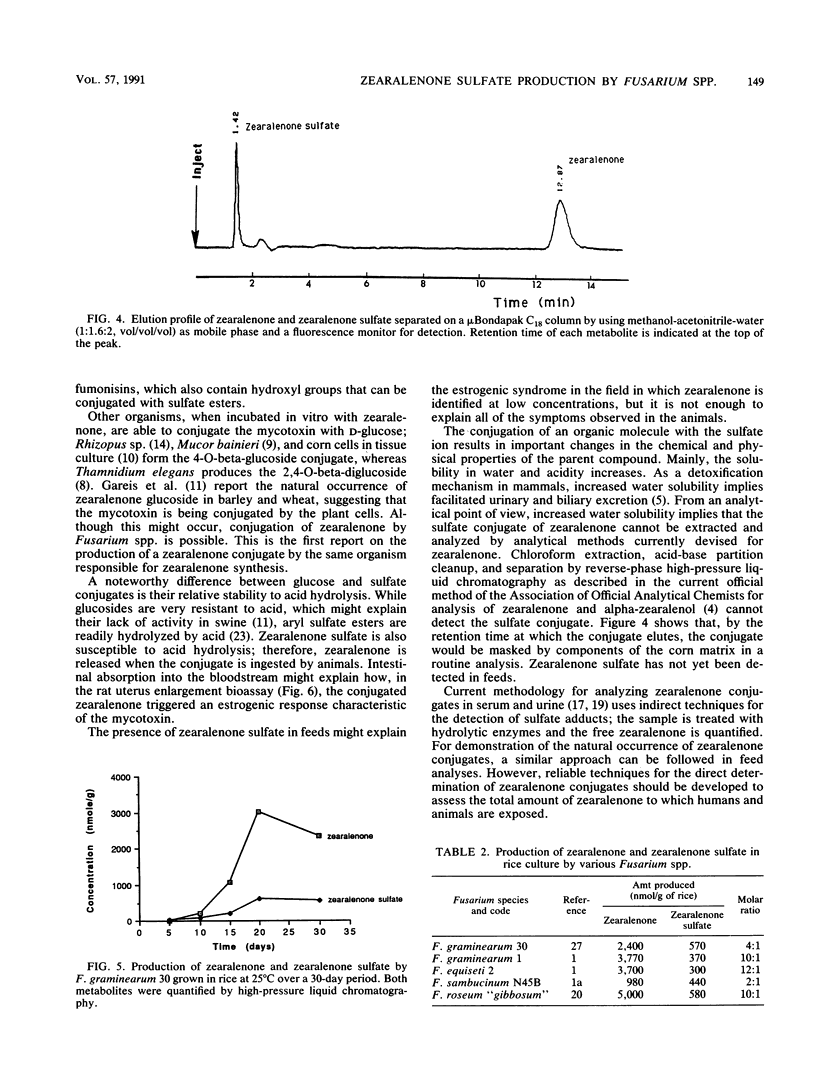
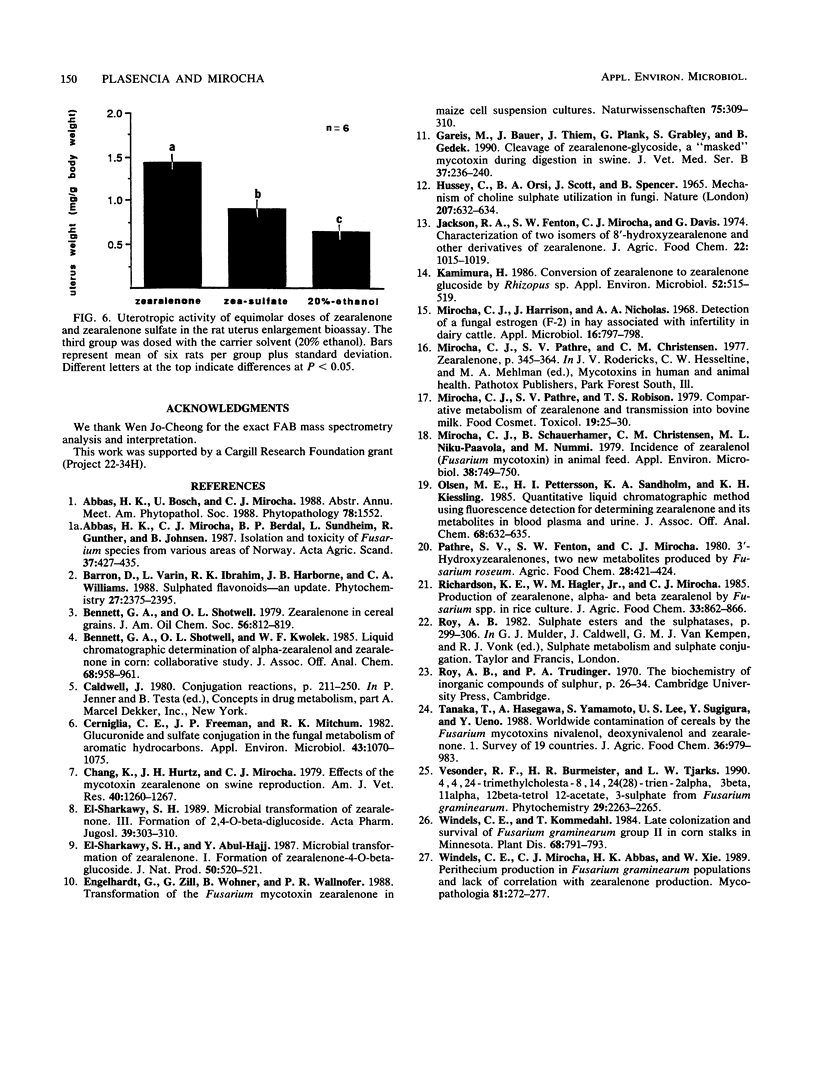
A water-soluble compound related to zearalenone was isolated from a culture of Fusarium graminearum 30 grown in rice. The structure of the novel metabolite was determined to be zearalenone-4-sulfate on the basis of fast-atom-bombardment mass spectrometry, proton nuclear magnetic resonance, UV spectroscopy, and by chemical and enzymatic reactions. Strains representing Fusarium equiseti, Fusarium sambucinum, and Fusarium roseum produced the sulfate conjugate as well. In the rat uterus enlargement bioassay, the metabolite or its hydrolysis product was found to retain the estrogenic activity characteristic of zearalenone. Natural occurrence of this novel metabolite might be significant because analytical methods devised for zearalenone in grain cannot detect the conjugate but the conjugate retains the biological properties of the mycotoxin when ingested by animals.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett G. A., Shotwell O. L., Kwolek W. F. Liquid chromatographic determination of alpha-zearalenol and zearalenone in corn: collaborative study. J Assoc Off Anal Chem. 1985 Sep-Oct;68(5):958–961. [PubMed] [Google Scholar]
- Cerniglia C. E., Freeman J. P., Mitchum R. K. Glucuronide and sulfate conjugation in the fungal metabolism of aromatic hydrocarbons. Appl Environ Microbiol. 1982 May;43(5):1070–1075. doi: 10.1128/aem.43.5.1070-1075.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K., Kurtz H. J., Mirocha C. J. Effects of the mycotoxin zearalenone on swine reproduction. Am J Vet Res. 1979 Sep;40(9):1260–1267. [PubMed] [Google Scholar]
- Engelhardt G., Zill G., Wohner B., Wallnöfer P. R. Transformation of the Fusarium mycotoxin zearalenone in maize cell suspension cultures. Naturwissenschaften. 1988 Jun;75(6):309–310. doi: 10.1007/BF00367324. [DOI] [PubMed] [Google Scholar]
- Gareis M., Bauer J., Thiem J., Plank G., Grabley S., Gedek B. Cleavage of zearalenone-glycoside, a "masked" mycotoxin, during digestion in swine. Zentralbl Veterinarmed B. 1990 May;37(3):236–240. doi: 10.1111/j.1439-0450.1990.tb01052.x. [DOI] [PubMed] [Google Scholar]
- Hussey C., Orsi B. A., Scott J., Spencer B. Mechanism of choline sulphate utilization in fungi. Nature. 1965 Aug 7;207(997):632–634. doi: 10.1038/207632b0. [DOI] [PubMed] [Google Scholar]
- Jackson R. A., Fenton S. W., Mirocha C. J., Davis G. Characterization of two isomers of 8'-hydroxyzearalenone and other derivatives of zearalenone. J Agric Food Chem. 1974 Nov-Dec;22(6):1015–1019. doi: 10.1021/jf60196a039. [DOI] [PubMed] [Google Scholar]
- Kamimura H. Conversion of zearalenone to zearalenone glycoside by Rhizopus sp. Appl Environ Microbiol. 1986 Sep;52(3):515–519. doi: 10.1128/aem.52.3.515-519.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirocha C. J., Harrison J., Nichols A. A., McClintock M. Detection of a fungal estrogen (F-2) in hay associated with infertility in dairy cattle. Appl Microbiol. 1968 May;16(5):797–798. doi: 10.1128/am.16.5.797-798.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirocha C. J., Pathre S. V., Robison T. S. Comparative metabolism of zearalenone and transmission into bovine milk. Food Cosmet Toxicol. 1981 Feb;19(1):25–30. doi: 10.1016/0015-6264(81)90299-6. [DOI] [PubMed] [Google Scholar]
- Mirocha C. J., Schauerhamer B., Christensen C. M., Niku-Paavola M. L., Nummi M. Incidence of zearalenol (Fusarium mycotoxin) in animal feed. Appl Environ Microbiol. 1979 Oct;38(4):749–750. doi: 10.1128/aem.38.4.749-750.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen M. E., Pettersson H. I., Sandholm K. A., Kiessling K. H. Quantitative liquid chromatographic method using fluorescence detection for determining zearalenone and its metabolites in blood plasma and urine. J Assoc Off Anal Chem. 1985 Jul-Aug;68(4):632–635. [PubMed] [Google Scholar]


