Abstract
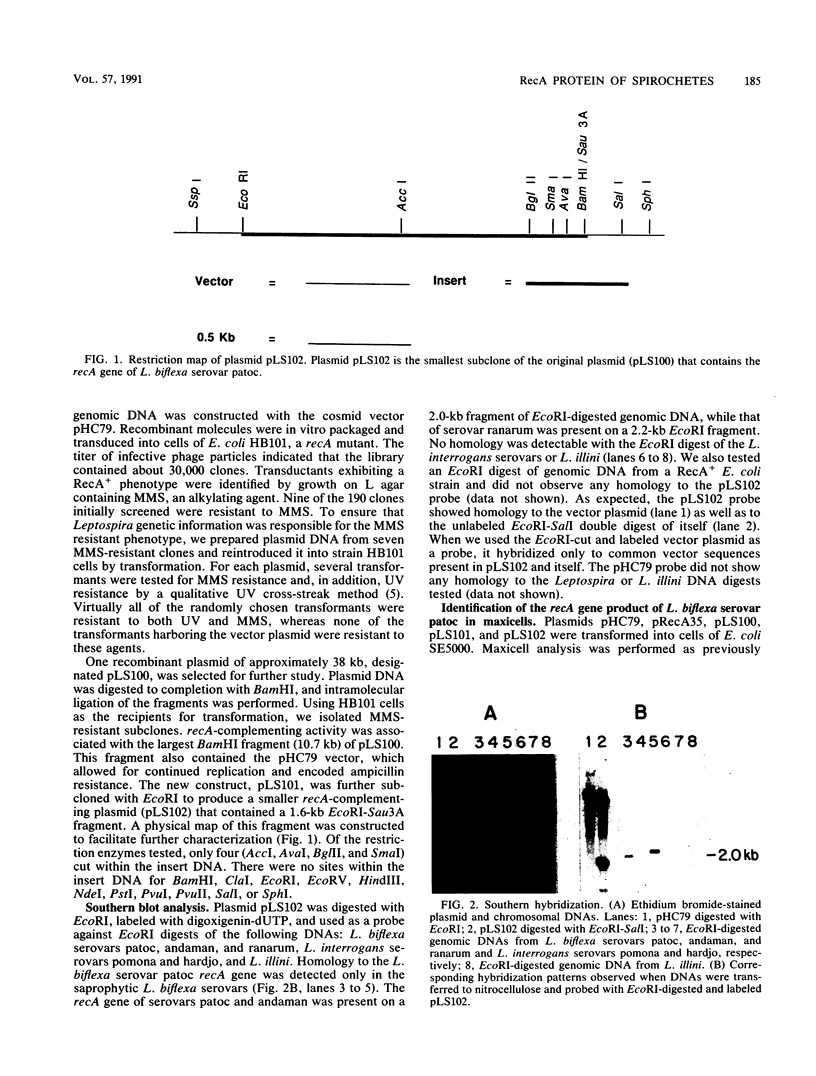
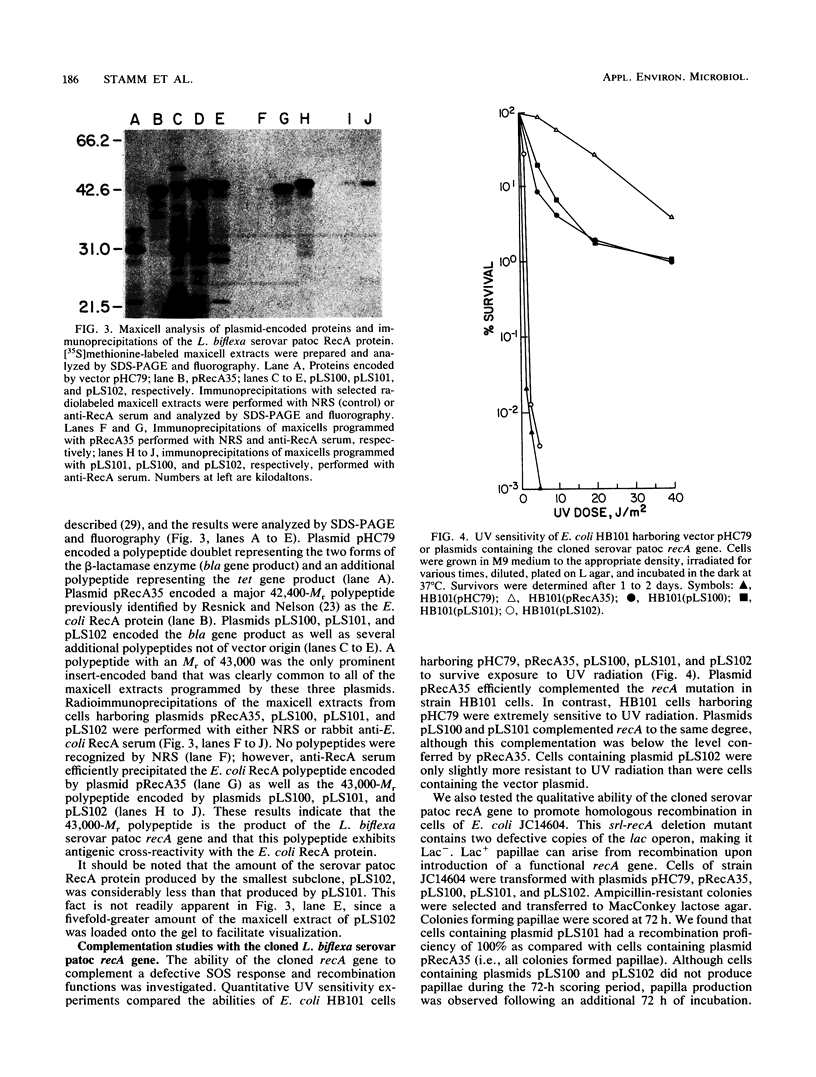
A recombinant plasmid carrying the recA gene of Leptospira biflexa serovar patoc was isolated from a cosmid library of genomic DNA by complementation of an Escherichia coli recA mutation. The cloned serovar patoc recA gene efficiently restored resistance to UV radiation and methyl methanesulfonate. Recombination proficiency was also restored, as measured by the formation of Lac+ recombinants from duplicated mutant lacZ genes. Additionally, the cloned recA gene increased the spontaneous and mitomycin C-induced production of lambda phage in lysogens of an E. coli recA mutant. The product of the cloned recA gene was identified in maxicells as a polypeptide with an Mr of 43,000. Antibodies prepared against the E. coli RecA protein cross-reacted with the serovar patoc RecA protein, indicating structural conservation. Southern hybridization data showed that the serovar patoc recA gene has diverged from the recA gene of L. interrogans, Leptonema illini, and E. coli. With the exception of the RecA protein of L. interrogans serovar hardjo, the RecA protein of the Leptospira serovars and L. illini were synthesized at elevated levels following treatment of cells with nalidixic acid. The level of detectable RecA correlated with previous studies demonstrating that free-living cells of L. biflexa serovars and L. illini were considerably more resistant to DNA-damaging agents than were those of parasitic L. interrogans serovars. RecA protein was not detected in cells of virulent Treponema pallidum or Borrelia burgdorferi.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfus L. A. Molecular cloning and expression in Escherichia coli of the recA gene of Legionella pneumophila. J Gen Microbiol. 1989 Nov;135(11):3097–3107. doi: 10.1099/00221287-135-11-3097. [DOI] [PubMed] [Google Scholar]
- ELLINGHAUSEN H. C., Jr, MCCULLOUGH W. G. NUTRITION OF LEPTOSPIRA POMONA AND GROWTH OF 13 OTHER SEROTYPES: A SERUM-FREE MEDIUM EMPLOYING OLEIC ALBUMIN COMPLEX. Am J Vet Res. 1965 Jan;26:39–44. [PubMed] [Google Scholar]
- Geoghegan C. M., Houghton J. A. Molecular cloning and isolation of a cyanobacterial gene which increases the UV and methyl methanesulphonate survival of recA strains of Escherichia coli K12. J Gen Microbiol. 1987 Jan;133(1):119–126. doi: 10.1099/00221287-133-1-119. [DOI] [PubMed] [Google Scholar]
- Gherardini F. C., Hobbs M. M., Stamm L. V., Bassford P. J., Jr Complementation of an Escherichia coli proC mutation by a gene cloned from Treponema pallidum. J Bacteriol. 1990 Jun;172(6):2996–3002. doi: 10.1128/jb.172.6.2996-3002.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg I., Mekalanos J. J. Cloning of the Vibrio cholerae recA gene and construction of a Vibrio cholerae recA mutant. J Bacteriol. 1986 Mar;165(3):715–722. doi: 10.1128/jb.165.3.715-722.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapala D. K., Rogul M., Evans L. B., Alexander A. D. Deoxyribonucleic acid base composition and homology studies of Leptospira. J Bacteriol. 1969 May;98(2):421–428. doi: 10.1128/jb.98.2.421-428.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamood A. N., Pettis G. S., Parker C. D., McIntosh M. A. Isolation and characterization of the Vibrio cholerae recA gene. J Bacteriol. 1986 Jul;167(1):375–378. doi: 10.1128/jb.167.1.375-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Harwood C. S., Canale-Parola E. Ecology of spirochetes. Annu Rev Microbiol. 1984;38:161–192. doi: 10.1146/annurev.mi.38.100184.001113. [DOI] [PubMed] [Google Scholar]
- Hickman M. J., Orser C. S., Willis D. K., Lindow S. E., Panopoulos N. J. Molecular cloning and biological characterization of the recA gene from Pseudomonas syringae. J Bacteriol. 1987 Jun;169(6):2906–2910. doi: 10.1128/jb.169.6.2906-2910.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Harris V. G. Differentiation of pathogenic and saprophytic letospires. I. Growth at low temperatures. J Bacteriol. 1967 Jul;94(1):27–31. doi: 10.1128/jb.94.1.27-31.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keener S. L., McNamee K. P., McEntee K. Cloning and characterization of recA genes froM Proteus vulgaris, Erwinia carotovora, Shigella flexneri, and Escherichia coli B/r. J Bacteriol. 1984 Oct;160(1):153–160. doi: 10.1128/jb.160.1.153-160.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokjohn T. A., Miller R. V. Molecular cloning and characterization of the recA gene of Pseudomonas aeruginosa PAO. J Bacteriol. 1985 Aug;163(2):568–572. doi: 10.1128/jb.163.2.568-572.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koomey J. M., Falkow S. Cloning of the recA gene of Neisseria gonorrhoeae and construction of gonococcal recA mutants. J Bacteriol. 1987 Feb;169(2):790–795. doi: 10.1128/jb.169.2.790-795.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. V., Kokjohn T. A. General microbiology of recA: environmental and evolutionary significance. Annu Rev Microbiol. 1990;44:365–394. doi: 10.1146/annurev.mi.44.100190.002053. [DOI] [PubMed] [Google Scholar]
- Nunes-Edwards P. L., Thiermann A. B., Bassford P. J., Jr, Stamm L. V. Identification and characterization of the protein antigens of Leptospira interrogans serovar hardjo. Infect Immun. 1985 May;48(2):492–497. doi: 10.1128/iai.48.2.492-497.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owttrim G. W., Coleman J. R. Molecular cloning of a recA-like gene from the cyanobacterium Anabaena variabilis. J Bacteriol. 1987 May;169(5):1824–1829. doi: 10.1128/jb.169.5.1824-1829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul K., Ghosh S. K., Das J. Cloning and expression in Escherichia coli of a recA-like gene from Vibrio cholerae. Mol Gen Genet. 1986 Apr;203(1):58–63. doi: 10.1007/BF00330384. [DOI] [PubMed] [Google Scholar]
- Resnick D., Nelson D. R. Cloning and characterization of the Aeromonas caviae recA gene and construction of an A. caviae recA mutant. J Bacteriol. 1988 Jan;170(1):48–55. doi: 10.1128/jb.170.1.48-55.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- Singer J. T. Molecular cloning of the recA analog from the marine fish pathogen Vibrio anguillarum 775. J Bacteriol. 1989 Nov;171(11):6367–6371. doi: 10.1128/jb.171.11.6367-6371.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. R. Homologous recombination in procaryotes. Microbiol Rev. 1988 Mar;52(1):1–28. doi: 10.1128/mr.52.1.1-28.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stamm L. V., Charon N. W. Sensitivity of pathogenic and free-living Leptospira spp. to UV radiation and mitomycin C. Appl Environ Microbiol. 1988 Mar;54(3):728–733. doi: 10.1128/aem.54.3.728-733.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm L. V., Kerner T. C., Jr, Bankaitis V. A., Bassford P. J., Jr Identification and preliminary characterization of Treponema pallidum protein antigens expressed in Escherichia coli. Infect Immun. 1983 Aug;41(2):709–721. doi: 10.1128/iai.41.2.709-721.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton J. T., Stamm L. V., Bassford P. J., Jr Potential for development of antibiotic resistance in pathogenic treponemes. Rev Infect Dis. 1985 May-Jun;7 (Suppl 2):S314–S317. doi: 10.1093/clinids/7-supplement_2.s314. [DOI] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelton D. B., Charon N. W. Cloning of a gene required for tryptophan biosynthesis from Leptospira biflexa serovar patoc into Escherichia coli. Gene. 1984 May;28(2):147–152. doi: 10.1016/0378-1119(84)90251-8. [DOI] [PubMed] [Google Scholar]
- Zuerner R. L., Charon N. W. Nucleotide sequence analysis of a gene cloned from Leptospira biflexa serovar patoc which complements an argE defect in Escherichia coli. J Bacteriol. 1988 Oct;170(10):4548–4554. doi: 10.1128/jb.170.10.4548-4554.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]