Abstract
Urea is reported to be a main precursor of ethyl carbamate (ECA), which is suspected to be a carcinogen, in wine and sake. In order to minimize production of urea, arginase-deficient mutants (delta car1/delta car1) were constructed from a diploid sake yeast, Kyokai no. 9, by successive disruption of the two copies of the CAR1 gene. First, the yeast strain was transformed with plasmid pCAT2 (delta car1 SMR1), and strains heterozygous for CAR1 gene were isolated on sulfometuron methyl plates. Successively, the other CAR1 gene was disrupted by transformation with plasmid pCAT1 (delta car1 G418r) and the resulting car1 mutants were isolated on a G418 plate. Arginase assay of the total cell lysate of the mutants showed that 70% of transformants isolated on G418 plates had no detectable enzyme activity, possibly as a result of the disruption of the two copies of the CAR1 gene. Further genomic Southern analysis confirmed this result. We could brew sake containing no urea with the delta car1/delta car1 homozygous mutant. It is of additional interest that no ECA was detected in the resulting sake, even after storage for 5 months at 30 degrees C. This molecular biological study suggests that ECA in sake originates mainly from urea that is produced by the arginase.
Full text
PDF
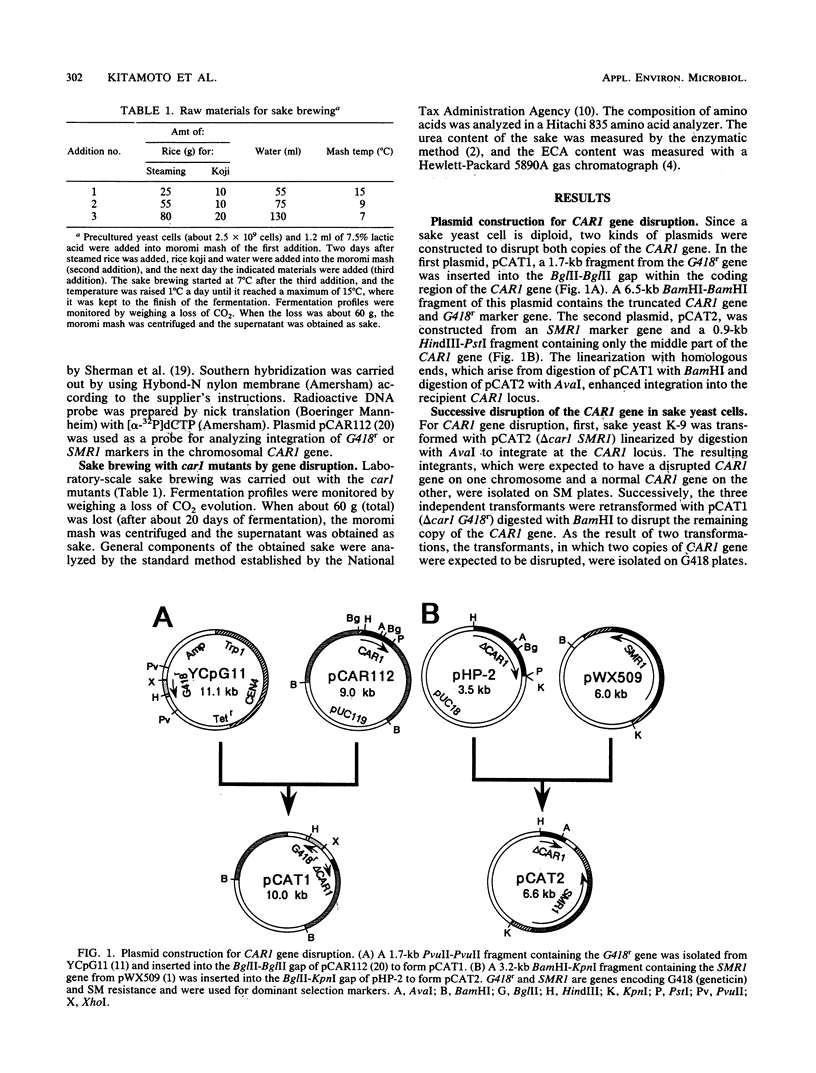
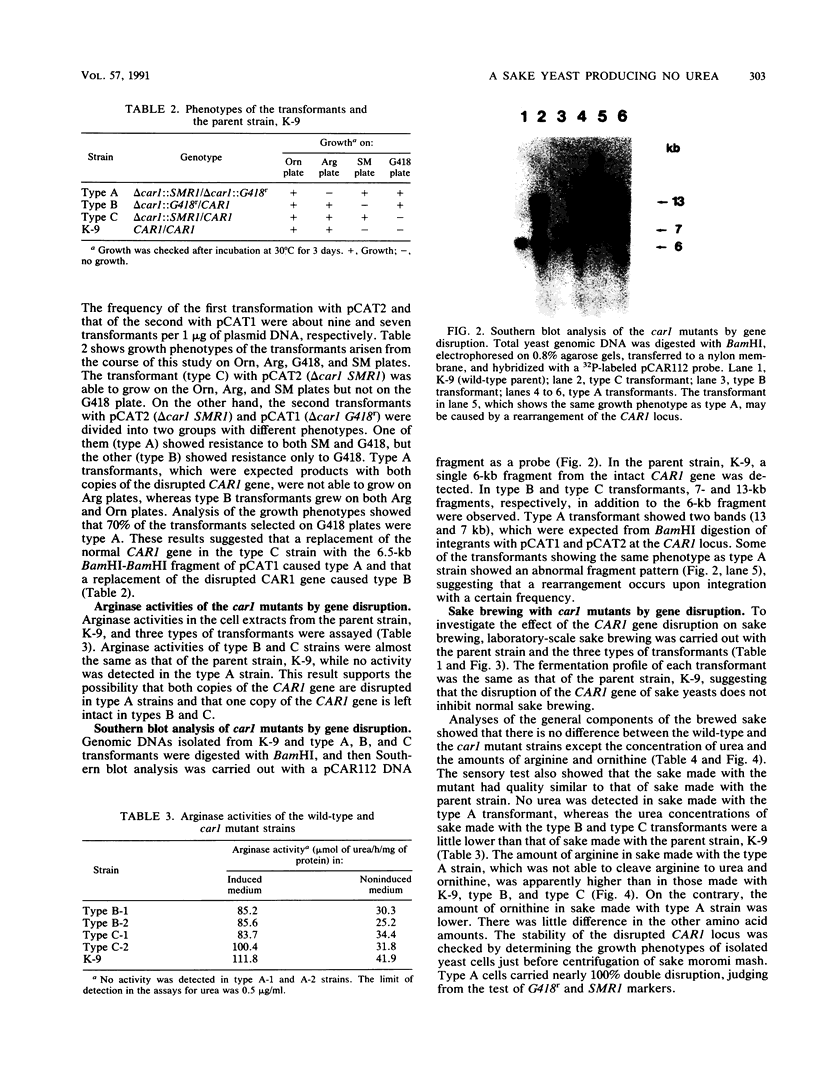
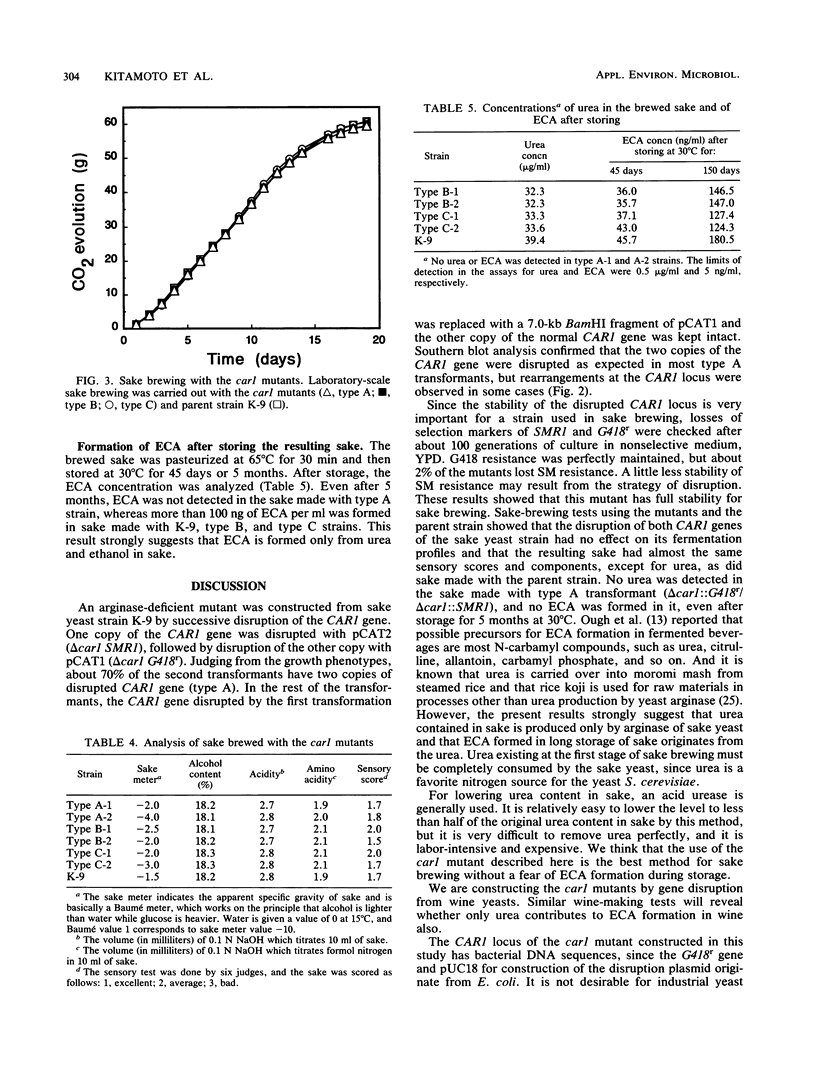

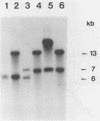
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIDDELHOVEN W. J. THE PATHWAY OF ARGININE BREAKDOWN IN SACCHAROMYCES CEREVISIAE. Biochim Biophys Acta. 1964 Dec 9;93:650–652. doi: 10.1016/0304-4165(64)90349-6. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mirvish S. S. The carcinogenic action and metabolism of urethan and N-hydroxyurethan. Adv Cancer Res. 1968;11:1–42. doi: 10.1016/s0065-230x(08)60386-3. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Miyamoto S., Ohsumi Y., Anraku Y. Calcium-sensitive cls4 mutant of Saccharomyces cerevisiae with a defect in bud formation. J Bacteriol. 1986 Jan;165(1):28–33. doi: 10.1128/jb.165.1.28-33.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ough C. S. Ethylcarbamate in fermented beverages and foods. I. Naturally occurring ethylcarbamate. J Agric Food Chem. 1976 Mar-Apr;24(2):323–328. doi: 10.1021/jf60204a033. [DOI] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Sumrada R. A., Cooper T. G. Isolation of the CAR1 gene from Saccharomyces cerevisiae and analysis of its expression. Mol Cell Biol. 1982 Dec;2(12):1514–1523. doi: 10.1128/mcb.2.12.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney P. A., Magasanik B. The induction of arginase in Saccharomyces cerevisiae. J Biol Chem. 1973 Sep 10;248(17):6197–6202. [PubMed] [Google Scholar]
- Winston F., Chumley F., Fink G. R. Eviction and transplacement of mutant genes in yeast. Methods Enzymol. 1983;101:211–228. doi: 10.1016/0076-6879(83)01016-2. [DOI] [PubMed] [Google Scholar]