Abstract
A cosmid gene library was constructed in Escherichia coli from genomic DNA isolated from the ruminal anaerobe Fibrobacter succinogenes AR1. Clones were screened on carboxymethyl cellulose, and 8 colonies that produced large clearing zones and 25 colonies that produced small clearing zones were identified. Southern blot hybridization revealed the existence of at least three separate genes encoding cellulase activity. pRC093, which is representative of cosmid clones that produce large clearing zones, was subcloned in pGem-1, and the resulting hybrid pRCEH directed synthesis of endoglucanase activity localized on a 2.1-kb EcoRI-HindIII insert. Activity was expressed from this fragment when it was cloned in both orientations in pGem-1 and pGem-2, indicating that F. succinogenes promoters functioned successfully in E. coli. A high level of endoglucanase activity was detected on acid-swollen cellulose, ball-milled cellulose, and carboxymethyl cellulose; and a moderate level was detected on filter paper, Avicel, lichenan, and xylan. Most activity (80%) was localized in the periplasm of E. coli, with low but significant levels (16%) being detected in the extracellular medium. The periplasmic endoglucanase had an estimated molecular weight of 46,500, had an optimum temperature of 39 degrees C, and exhibited activity over a broad pH range, with a maximum at pH 5.0.
Full text
PDF


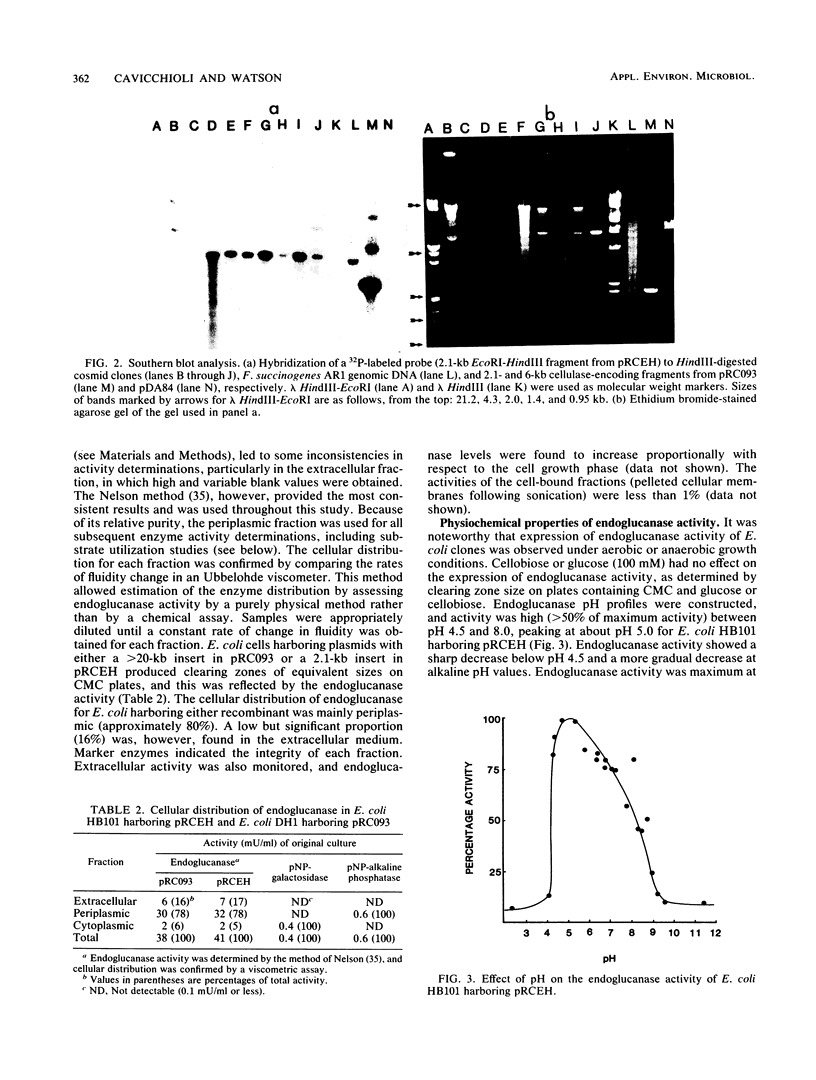



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann R. I., Krumholz L., Stahl D. A. Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J Bacteriol. 1990 Feb;172(2):762–770. doi: 10.1128/jb.172.2.762-770.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. G., McKay L. L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983 Sep;46(3):549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J. Plasmid vectors for the selection of promoters. Gene. 1984 Feb;27(2):151–160. doi: 10.1016/0378-1119(84)90136-7. [DOI] [PubMed] [Google Scholar]
- Erfle J. D., Teather R. M., Wood P. J., Irvin J. E. Purification and properties of a 1,3-1,4-beta-D-glucanase (lichenase, 1,3-1,4-beta-D-glucan 4-glucanohydrolase, EC 3.2.1.73) from Bacteroides succinogenes cloned in Escherichia coli. Biochem J. 1988 Nov 1;255(3):833–841. doi: 10.1042/bj2550833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson K. E., Hollmark B. H. Kinetic studies of the action of cellulase upon sodium carboxymethyl cellulose. Arch Biochem Biophys. 1969 Sep;133(2):233–237. doi: 10.1016/0003-9861(69)90450-0. [DOI] [PubMed] [Google Scholar]
- Gilardi G. L. Characterization of nonfermentative nonfastidious gram negative bacteria encountered in medical bacteriology. J Appl Bacteriol. 1971 Sep;34(3):623–644. doi: 10.1111/j.1365-2672.1971.tb02326.x. [DOI] [PubMed] [Google Scholar]
- Gilkes N. R., Langsford M. L., Kilburn D. G., Miller R. C., Jr, Warren R. A. Mode of action and substrate specificities of cellulases from cloned bacterial genes. J Biol Chem. 1984 Aug 25;259(16):10455–10459. [PubMed] [Google Scholar]
- Groleau D., Forsberg C. W. Cellulolytic activity of the rumen bacterium Bacteroides succinogenes. Can J Microbiol. 1981 May;27(5):517–530. doi: 10.1139/m81-077. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hohn B., Collins J. A small cosmid for efficient cloning of large DNA fragments. Gene. 1980 Nov;11(3-4):291–298. doi: 10.1016/0378-1119(80)90069-4. [DOI] [PubMed] [Google Scholar]
- Howard G. T., White B. A. Molecular Cloning and Expression of Cellulase Genes from Ruminococcus albus 8 in Escherichia coli Bacteriophage lambda. Appl Environ Microbiol. 1988 Jul;54(7):1752–1755. doi: 10.1128/aem.54.7.1752-1755.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Forsberg C. W. Isolation of a Cellodextrinase from Bacteroides succinogenes. Appl Environ Microbiol. 1987 May;53(5):1034–1041. doi: 10.1128/aem.53.5.1034-1041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., McGavin M., Forsberg C. W., Lam J. S., Cheng K. J. Antigenic nature of the chloride-stimulated cellobiosidase and other cellulases of Fibrobacter succinogenes subsp. succinogenes S85 and related fresh isolates. Appl Environ Microbiol. 1990 May;56(5):1229–1234. doi: 10.1128/aem.56.5.1229-1234.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin J. E., Teather R. M. Cloning and expression of a Bacteroides succinogenes mixed-linkage beta-glucanase (1,3-1,4-beta-D-glucan 4-glucanohydrolase) gene in Escherichia coli. Appl Environ Microbiol. 1988 Nov;54(11):2672–2676. doi: 10.1128/aem.54.11.2672-2676.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermid K. P., Mackenzie C. R., Forsberg C. W. Esterase Activities of Fibrobacter succinogenes subsp. succinogenes S85. Appl Environ Microbiol. 1990 Jan;56(1):127–132. doi: 10.1128/aem.56.1.127-132.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavin M. J., Forsberg C. W., Crosby B., Bell A. W., Dignard D., Thomas D. Y. Structure of the cel-3 gene from Fibrobacter succinogenes S85 and characteristics of the encoded gene product, endoglucanase 3. J Bacteriol. 1989 Oct;171(10):5587–5595. doi: 10.1128/jb.171.10.5587-5595.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavin M., Forsberg C. W. Isolation and characterization of endoglucanases 1 and 2 from Bacteroides succinogenes S85. J Bacteriol. 1988 Jul;170(7):2914–2922. doi: 10.1128/jb.170.7.2914-2922.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NATH R. L., RYDON H. N. The influence of structure on the hydrolysis of substituted phenyl beta-D-glucosides by emulsin. Biochem J. 1954 May;57(1):1–10. doi: 10.1042/bj0570001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Ohmiya K., Nagashima K., Kajino T., Goto E., Tsukada A., Shimizu S. Cloning of the cellulase gene from Ruminococcus albus and its expression in Escherichia coli. Appl Environ Microbiol. 1988 Jun;54(6):1511–1515. doi: 10.1128/aem.54.6.1511-1515.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney S. D., Benkovic S. J., Schimmel P. R. A DNA fragment with an alpha-phosphorothioate nucleotide at one end is asymmetrically blocked from digestion by exonuclease III and can be replicated in vivo. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7350–7354. doi: 10.1073/pnas.78.12.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romaniec M. P., Clarke N. G., Hazlewood G. P. Molecular cloning of Clostridium thermocellum DNA and the expression of further novel endo-beta-1,4-glucanase genes in Escherichia coli. J Gen Microbiol. 1987 May;133(5):1297–1307. doi: 10.1099/00221287-133-5-1297. [DOI] [PubMed] [Google Scholar]
- Sipat A., Taylor K. A., Lo R. Y., Forsberg C. W., Krell P. J. Molecular cloning of a xylanase gene from Bacteroides succinogenes and its expression in Escherichia coli. Appl Environ Microbiol. 1987 Mar;53(3):477–481. doi: 10.1128/aem.53.3.477-481.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stewart C. S., Paniagua C., Dinsdale D., Cheng K. J., Garrow S. H. Selective isolation and characteristics of Bacteriodes succinogenes from the rumen of a cow. Appl Environ Microbiol. 1981 Feb;41(2):504–510. doi: 10.1128/aem.41.2.504-510.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K. A., Crosby B., McGavin M., Forsberg C. W., Thomas D. Y. Characteristics of the endoglucanase encoded by a cel gene from Bacteroides succinogenes expressed in Escherichia coli. Appl Environ Microbiol. 1987 Jan;53(1):41–46. doi: 10.1128/aem.53.1.41-46.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teather R. M., Wood P. J. Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol. 1982 Apr;43(4):777–780. doi: 10.1128/aem.43.4.777-780.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware C. E., Bauchop T., Gregg K. The isolation and comparison of cellulase genes from two strains of Ruminococcus albus. J Gen Microbiol. 1989 Apr;135(4):921–930. doi: 10.1099/00221287-135-4-921. [DOI] [PubMed] [Google Scholar]
- Wood T. M. The cellulase of Fusarium solani. Purification and specificity of the -(1-4)-glucanase and the -D-glucosidase components. Biochem J. 1971 Feb;121(3):353–362. doi: 10.1042/bj1210353. [DOI] [PMC free article] [PubMed] [Google Scholar]



