Abstract
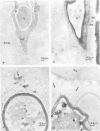
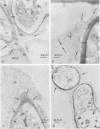
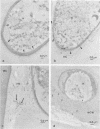
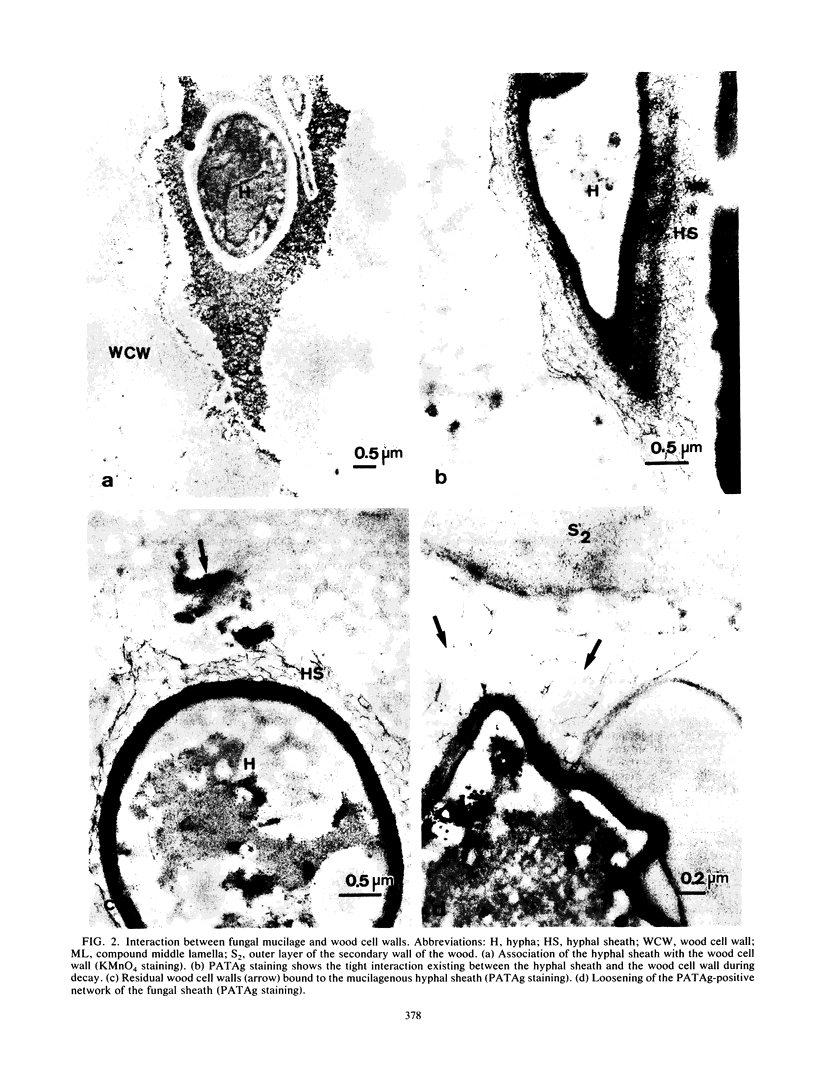
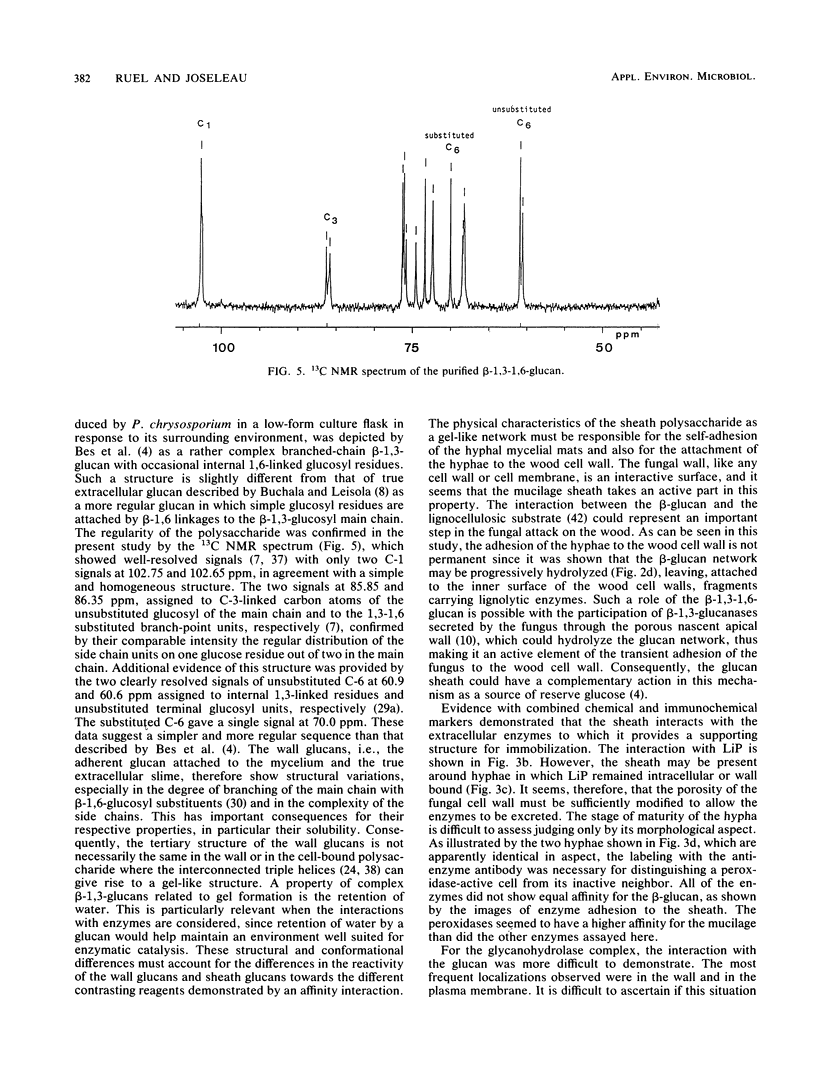
Observations by transmission electron microscopy of wood samples of Populus tremula inoculated with the white rot fungus Phanerochaete chrysosporium showed that, at certain stages of their growth cycle, hyphae were encapsulated by a sheath which seems to play an active role in the wood cell wall degradation. Chemical and immunochemical techniques and 13C nuclear magnetic resonance spectroscopy were applied to demonstrate the β-1,3-1,6-d-glucan nature of the sheath. Double-staining methods revealed the interaction between the extracellular peroxidases involved in lignin degradation and the glucan mucilage. The glucan was also shown to establish a material junction between the fungus and the wood cell wall. It was concluded that, by means of these interactions, the sheath provides a transient junction between the hyphae and the wood, thus establishing a point of attachment to the site of the degradation. The association of peroxidases to the glucan matrix is in favor of the role of the sheath as a supporting structure. Furthermore, that the sheath was hydrolyzed during the attack demonstrated its active role both in providing the H2O2 necessary to the action of peroxidases and in providing a mode of transport of the fungal enzymes to their substrates at the surface of the wood cell wall.
Full text
PDF

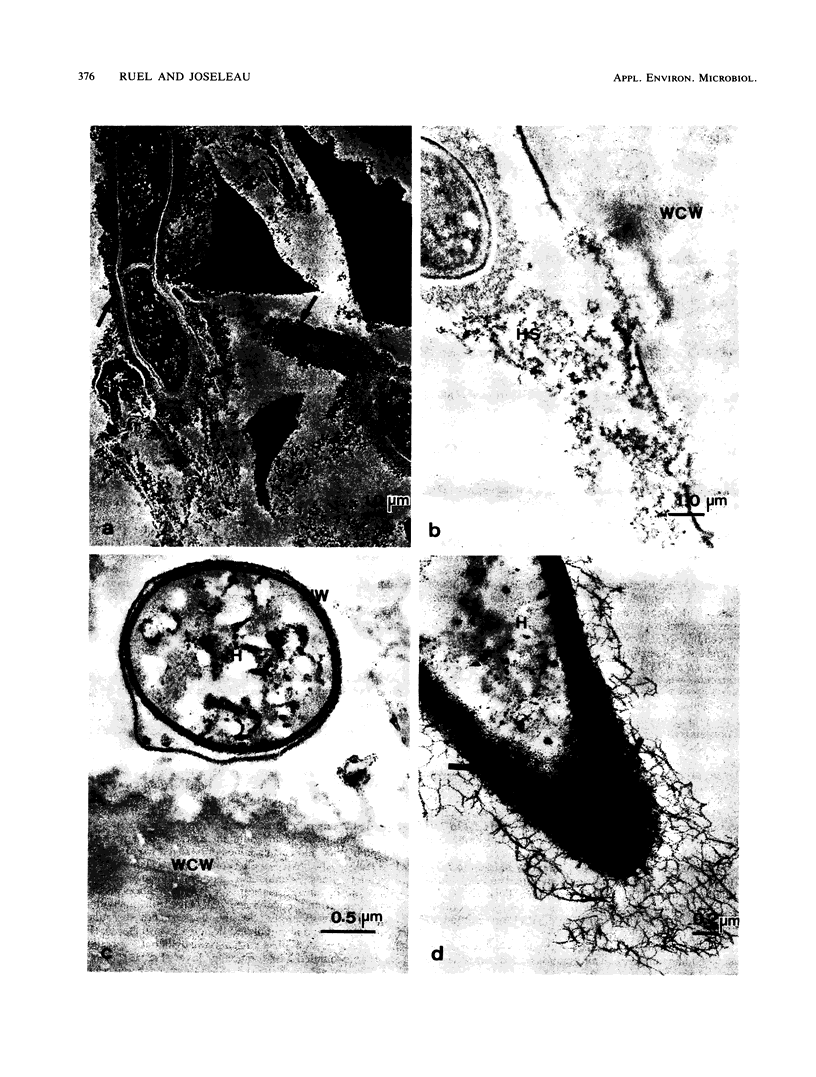






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson-Prouty A. J., Albersheim P. Host-Pathogen Interactions: VIII. Isolation of a Pathogen-synthesized Fraction Rich in Glucan That Elicits a Defense Response in the Pathogen's Host. Plant Physiol. 1975 Aug;56(2):286–291. doi: 10.1104/pp.56.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnicki-Garcia S. Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu Rev Microbiol. 1968;22:87–108. doi: 10.1146/annurev.mi.22.100168.000511. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bruneteau M., Fabre I., Perret J., Michel G., Ricci P., Joseleau J. P., Kraus J., Schneider M., Blaschek W., Franz G. Antitumor active beta-D-glucans from Phytophthora parasitica. Carbohydr Res. 1988 Apr 1;175(1):137–143. doi: 10.1016/0008-6215(88)80164-2. [DOI] [PubMed] [Google Scholar]
- Chang P. L., Trevithick J. R. How important is secretion of exoenzymes through apical cell walls of fungi? Arch Microbiol. 1974;101(4):281–293. doi: 10.1007/BF00455945. [DOI] [PubMed] [Google Scholar]
- Daniel G., Nilsson T., Pettersson B. Intra- and Extracellular Localization of Lignin Peroxidase during the Degradation of Solid Wood and Wood Fragments by Phanerochaete chrysosporium by Using Transmission Electron Microscopy and Immuno-Gold Labeling. Appl Environ Microbiol. 1989 Apr;55(4):871–881. doi: 10.1128/aem.55.4.871-881.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch F., Staehelin L. A. Functional Implications of the Subcellular Localization of Ethylene-Induced Chitinase and [beta]-1,3-Glucanase in Bean Leaves. Plant Cell. 1989 Apr;1(4):447–457. doi: 10.1105/tpc.1.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niku-Paavola M. L., Karhunen E., Salola P., Raunio V. Ligninolytic enzymes of the white-rot fungus Phlebia radiata. Biochem J. 1988 Sep 15;254(3):877–883. doi: 10.1042/bj2540877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Saloheimo M., Barajas V., Niku-Paavola M. L., Knowles J. K. A lignin peroxidase-encoding cDNA from the white-rot fungus Phlebia radiata: characterization and expression in Trichoderma reesei. Gene. 1989 Dec 28;85(2):343–351. doi: 10.1016/0378-1119(89)90427-7. [DOI] [PubMed] [Google Scholar]
- Sietsma J. H., Wessels J. G. Solubility of (1 leads to 3)-beta-D/(1 leads to 6)-beta-D-glucan in fungal walls: importance of presumed linkage between glucan and chitin. J Gen Microbiol. 1981 Jul;125(1):209–212. doi: 10.1099/00221287-125-1-209. [DOI] [PubMed] [Google Scholar]
- Spaur R. C., Moriarty G. C. Improvements of glycol methacrylate. I. Its use as an embedding medium for electron microscopic studies. J Histochem Cytochem. 1977 Mar;25(3):163–174. doi: 10.1177/25.3.839061. [DOI] [PubMed] [Google Scholar]
- Whistler R. L., Bushway A. A., Singh P. P. Noncytotoxic, antitumor polysaccharides. Adv Carbohydr Chem Biochem. 1976;32:235–275. doi: 10.1016/s0065-2318(08)60338-8. [DOI] [PubMed] [Google Scholar]