Abstract
The genetic structure of a population of nonsymbiotic Rhizobium leguminosarum strains was determined by the electrophoretic mobilities of eight metabolic enzymes. Nonsymbiotic strains were isolated from the rhizosphere of bean plants and characterized by growth on differential media and at different temperatures, intrinsic antibiotic resistance, the lack of homology to a nifH probe, and their inability to form nodules on bean roots. All the isolates clustered with R. leguminosarum bv. phaseoli reference strains and did not encompass any other Rhizobium taxa. Their rRNA operon restriction fragment length polymorphisms and the nucleotide sequence of a fragment of the 16S rRNA gene were also found to be identical to those of R. leguminosarum bv. phaseoli reference strains. When complemented with an R. leguminosarum bv. phaseoli symbiotic plasmid (p42d), the nonsymbiotic isolates were able to fix nitrogen in symbiosis with bean roots at levels similar to those of the parental strain. The symbiotic isolates were found at a relative frequency of 1 in 40 nonsymbiotic R. leguminosarum strains.
Full text
PDF

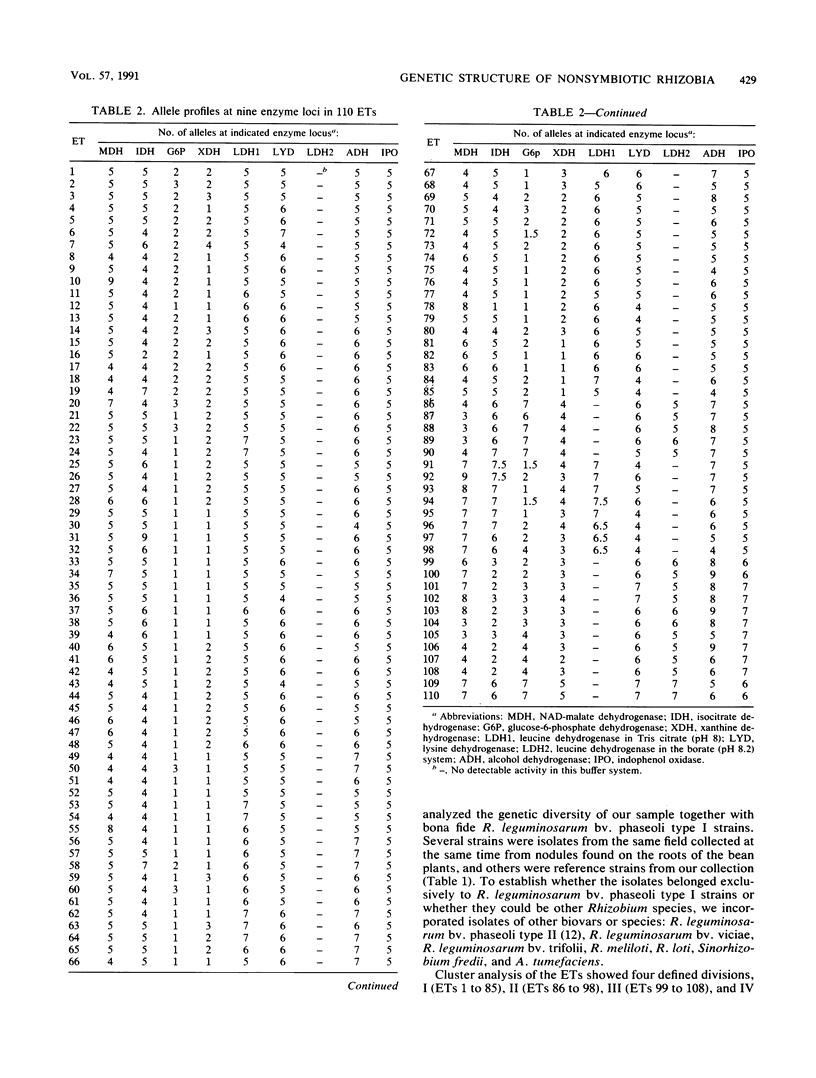
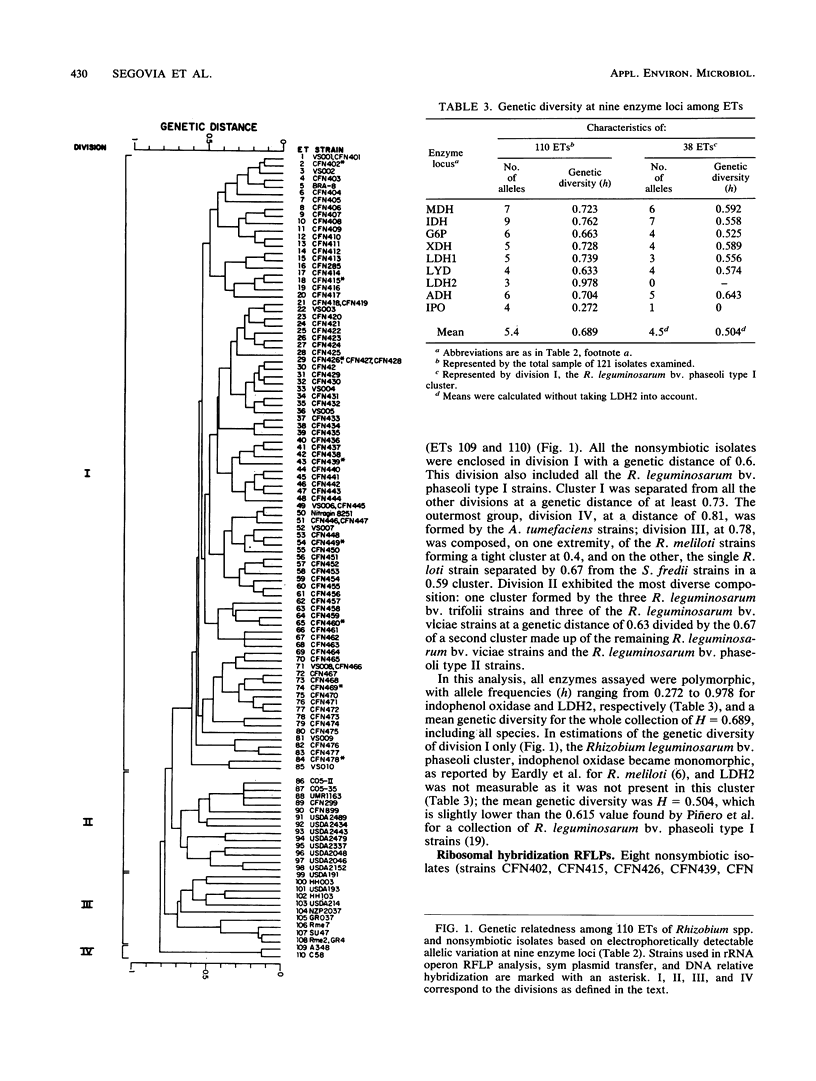


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Brom S., Martinez E., Dávila G., Palacios R. Narrow- and Broad-Host-Range Symbiotic Plasmids of Rhizobium spp. Strains That Nodulate Phaseolus vulgaris. Appl Environ Microbiol. 1988 May;54(5):1280–1283. doi: 10.1128/aem.54.5.1280-1283.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Casanova J. L., Pannetier C., Jaulin C., Kourilsky P. Optimal conditions for directly sequencing double-stranded PCR products with sequenase. Nucleic Acids Res. 1990 Jul 11;18(13):4028–4028. doi: 10.1093/nar/18.13.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eardly B. D., Materon L. A., Smith N. H., Johnson D. A., Rumbaugh M. D., Selander R. K. Genetic structure of natural populations of the nitrogen-fixing bacterium Rhizobium meliloti. Appl Environ Microbiol. 1990 Jan;56(1):187–194. doi: 10.1128/aem.56.1.187-194.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores M., González V., Pardo M. A., Leija A., Martínez E., Romero D., Piñero D., Dávila G., Palacios R. Genomic instability in Rhizobium phaseoli. J Bacteriol. 1988 Mar;170(3):1191–1196. doi: 10.1128/jb.170.3.1191-1196.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis B. D., Ward L. J., Slade E. A. Expression by Soil Bacteria of Nodulation Genes from Rhizobium leguminosarum biovar trifolii. Appl Environ Microbiol. 1989 Jun;55(6):1426–1434. doi: 10.1128/aem.55.6.1426-1434.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaijalainen S., Lindström K. Restriction fragment length polymorphism analysis of Rhizobium galegae strains. J Bacteriol. 1989 Oct;171(10):5561–5566. doi: 10.1128/jb.171.10.5561-5566.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez E., Palacios R., Sánchez F. Nitrogen-fixing nodules induced by Agrobacterium tumefaciens harboring Rhizobium phaseoli plasmids. J Bacteriol. 1987 Jun;169(6):2828–2834. doi: 10.1128/jb.169.6.2828-2834.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neefs J. M., Van de Peer Y., Hendriks L., De Wachter R. Compilation of small ribosomal subunit RNA sequences. Nucleic Acids Res. 1990 Apr 25;18 (Suppl):2237–2317. doi: 10.1093/nar/18.suppl.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M., Li W. H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel K. D., Sanchez A., Fernandez L., Leemans J., Cevallos M. A. Rhizobium phaseoli symbiotic mutants with transposon Tn5 insertions. J Bacteriol. 1984 Apr;158(1):148–155. doi: 10.1128/jb.158.1.148-155.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinero D., Martinez E., Selander R. K. Genetic diversity and relationships among isolates of Rhizobium leguminosarum biovar phaseoli. Appl Environ Microbiol. 1988 Nov;54(11):2825–2832. doi: 10.1128/aem.54.11.2825-2832.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinto C., De La Vega H., Flores M., Leemans J., Cevallos M. A., Pardo M. A., Azpiroz R., De Lourdes Girard M., Calva E., Palacios R. Nitrogenase reductase: A functional multigene family in Rhizobium phaseoli. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1170–1174. doi: 10.1073/pnas.82.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield P. R., Gibson A. H., Dudman W. F., Watson J. M. Evidence for genetic exchange and recombination of Rhizobium symbiotic plasmids in a soil population. Appl Environ Microbiol. 1987 Dec;53(12):2942–2947. doi: 10.1128/aem.53.12.2942-2947.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander R. K., Caugant D. A., Ochman H., Musser J. M., Gilmour M. N., Whittam T. S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986 May;51(5):873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R. High frequency mobilization of gram-negative bacterial replicons by the in vitro constructed Tn5-Mob transposon. Mol Gen Genet. 1984;196(3):413–420. doi: 10.1007/BF00436188. [DOI] [PubMed] [Google Scholar]
- Soberón-Chávez G., Nájera R., Olivera H., Segovia L. Genetic rearrangements of a Rhizobium phaseoli symbiotic plasmid. J Bacteriol. 1986 Aug;167(2):487–491. doi: 10.1128/jb.167.2.487-491.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]