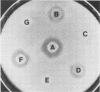
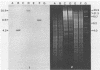
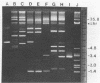
Abstract
Conjugation was used to construct nisin-producing Lactococcus lactis subsp. cremoris strains. Recipients were obtained by electroporation of L. lactis subsp. cremoris strains with the drug resistance plasmid pGK13 or pGB301. A method, direct-plate conjugation, was developed in which donor and recipient cells were concentrated and then combined directly on selective media. This method facilitated transfer of the nisin-sucrose (Nip+ Suc+) phenotype from the donor strain, L. lactis subsp. lactis 11454, to three L. lactis subsp. cremoris recipient strains. Nip+ Suc+ L. lactis subsp. cremoris transconjugants were obtained at frequencies which ranged from 10(-7) to 10(-8) per donor CFU. DNA-DNA hybridization to transconjugant DNAs, performed with an oligonucleotide probe synthesized to detect the nisin precursor gene, showed that this gene was transferred during conjugation but was not associated with detectable plasmid DNA. Further investigation indicated that L. lactis subsp. cremoris Nip+ Suc+ transconjugants retained the recipient strain phenotype with respect to bacteriophage resistance and acid production in milk. Results suggested that it would be feasible to construct nisin-producing L. lactis subsp. cremoris strains for application as mixed and multiple starter systems. Additionally, the direct-plate conjugation method required less time than filter or milk agar matings and may also be useful for investigations of conjugal mechanisms in these organisms.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. G., McKay L. L. Genetic and physical characterization of recombinant plasmids associated with cell aggregation and high-frequency conjugal transfer in Streptococcus lactis ML3. J Bacteriol. 1984 Jun;158(3):954–962. doi: 10.1128/jb.158.3.954-962.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. G., McKay L. L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983 Sep;46(3):549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke D., Gilmore M. S., Ferretti J. J. Plasmid pGB301, a new multiple resistance streptococcal cloning vehicle and its use in cloning of a gentamicin/kanamycin resistance determinant. Mol Gen Genet. 1981;182(3):414–421. doi: 10.1007/BF00293929. [DOI] [PubMed] [Google Scholar]
- Benkerroum N., Sandine W. E. Inhibitory action of nisin against Listeria monocytogenes. J Dairy Sci. 1988 Dec;71(12):3237–3245. doi: 10.3168/jds.S0022-0302(88)79929-4. [DOI] [PubMed] [Google Scholar]
- Buchman G. W., Banerjee S., Hansen J. N. Structure, expression, and evolution of a gene encoding the precursor of nisin, a small protein antibiotic. J Biol Chem. 1988 Nov 5;263(31):16260–16266. [PubMed] [Google Scholar]
- Dodd H. M., Horn N., Gasson M. J. Analysis of the genetic determinant for production of the peptide antibiotic nisin. J Gen Microbiol. 1990 Mar;136(3):555–566. doi: 10.1099/00221287-136-3-555. [DOI] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald G. F., Gasson M. J. In vivo gene transfer systems and transposons. Biochimie. 1988 Apr;70(4):489–502. doi: 10.1016/0300-9084(88)90085-5. [DOI] [PubMed] [Google Scholar]
- Franke A. E., Clewell D. B. Evidence for a chromosome-borne resistance transposon (Tn916) in Streptococcus faecalis that is capable of "conjugal" transfer in the absence of a conjugative plasmid. J Bacteriol. 1981 Jan;145(1):494–502. doi: 10.1128/jb.145.1.494-502.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasson M. J., Davies F. L. High-frequency conjugation associated with Streptococcus lactis donor cell aggregation. J Bacteriol. 1980 Sep;143(3):1260–1264. doi: 10.1128/jb.143.3.1260-1264.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C. F., Kunka B. S. Transfer of Sucrose-Fermenting Ability and Nisin Production Phenotype among Lactic Streptococci. Appl Environ Microbiol. 1985 Mar;49(3):627–633. doi: 10.1128/aem.49.3.627-633.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershfield V. Plasmids mediating multiple drug resistance in group B streptococcus: transferability and molecular properties. Plasmid. 1979 Jan;2(1):137–149. doi: 10.1016/0147-619x(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Holo H., Nes I. F. High-Frequency Transformation, by Electroporation, of Lactococcus lactis subsp. cremoris Grown with Glycine in Osmotically Stabilized Media. Appl Environ Microbiol. 1989 Dec;55(12):3119–3123. doi: 10.1128/aem.55.12.3119-3123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaletta C., Entian K. D. Nisin, a peptide antibiotic: cloning and sequencing of the nisA gene and posttranslational processing of its peptide product. J Bacteriol. 1989 Mar;171(3):1597–1601. doi: 10.1128/jb.171.3.1597-1601.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Walsh P. M. Conjugal transfer of genetic information in group N streptococci. Appl Environ Microbiol. 1980 Jul;40(1):84–89. doi: 10.1128/aem.40.1.84-91.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Zottola E. A. Loss of lactose metabolism in lactic streptococci. Appl Microbiol. 1972 Jun;23(6):1090–1096. doi: 10.1128/am.23.6.1090-1096.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. C., Steele J. L., Daly C., McKay L. L. Concomitant conjugal transfer of reduced-bacteriophage-sensitivity mechanisms with lactose- and sucrose-fermenting ability in lactic streptococci. Appl Environ Microbiol. 1988 Aug;54(8):1951–1956. doi: 10.1128/aem.54.8.1951-1956.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell Ian B., Achen Marc G., Hillier Alan J., Davidson Barrie E. A Simple and Rapid Method for Genetic Transformation of Lactic Streptococci by Electroporation. Appl Environ Microbiol. 1988 Mar;54(3):655–660. doi: 10.1128/aem.54.3.655-660.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders M. E., Leonhard P. J., Sing W. D., Klaenhammer T. R. Conjugal strategy for construction of fast Acid-producing, bacteriophage-resistant lactic streptococci for use in dairy fermentations. Appl Environ Microbiol. 1986 Nov;52(5):1001–1007. doi: 10.1128/aem.52.5.1001-1007.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snook R. J., McKay L. L. Conjugal Transfer of Lactose-Fermenting Ability Among Streptococcus cremoris and Streptococcus lactis Strains. Appl Environ Microbiol. 1981 Nov;42(5):904–911. doi: 10.1128/aem.42.5.904-911.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steele J. L., McKay L. L. Partial characterization of the genetic basis for sucrose metabolism and nisin production in Streptococcus lactis. Appl Environ Microbiol. 1986 Jan;51(1):57–64. doi: 10.1128/aem.51.1.57-64.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenson L. R., Klaenhammer T. R. Conjugal transfer of plasmid DNA between streptococci immobilized in calcium alginate gel beads. Appl Environ Microbiol. 1987 Apr;53(4):898–900. doi: 10.1128/aem.53.4.898-900.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H. J., Sandine W. E. Conjugal transfer of nisin plasmid genes from Streptococcus lactis 7962 to Leuconostoc dextranicum 181. Appl Environ Microbiol. 1987 Feb;53(2):352–357. doi: 10.1128/aem.53.2.352-357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh P. M., McKay L. L. Recombinant plasmid associated cell aggregation and high-frequency conjugation of Streptococcus lactis ML3. J Bacteriol. 1981 Jun;146(3):937–944. doi: 10.1128/jb.146.3.937-944.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N., Wilkins B. Processing of plasmid DNA during bacterial conjugation. Microbiol Rev. 1984 Mar;48(1):24–41. doi: 10.1128/mr.48.1.24-41.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woskow S. A., Kondo J. K. Effect of Proteolytic Enzymes on Transfection and Transformation of Streptococcus lactis Protoplasts. Appl Environ Microbiol. 1987 Oct;53(10):2583–2587. doi: 10.1128/aem.53.10.2583-2587.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]