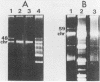
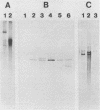
Abstract
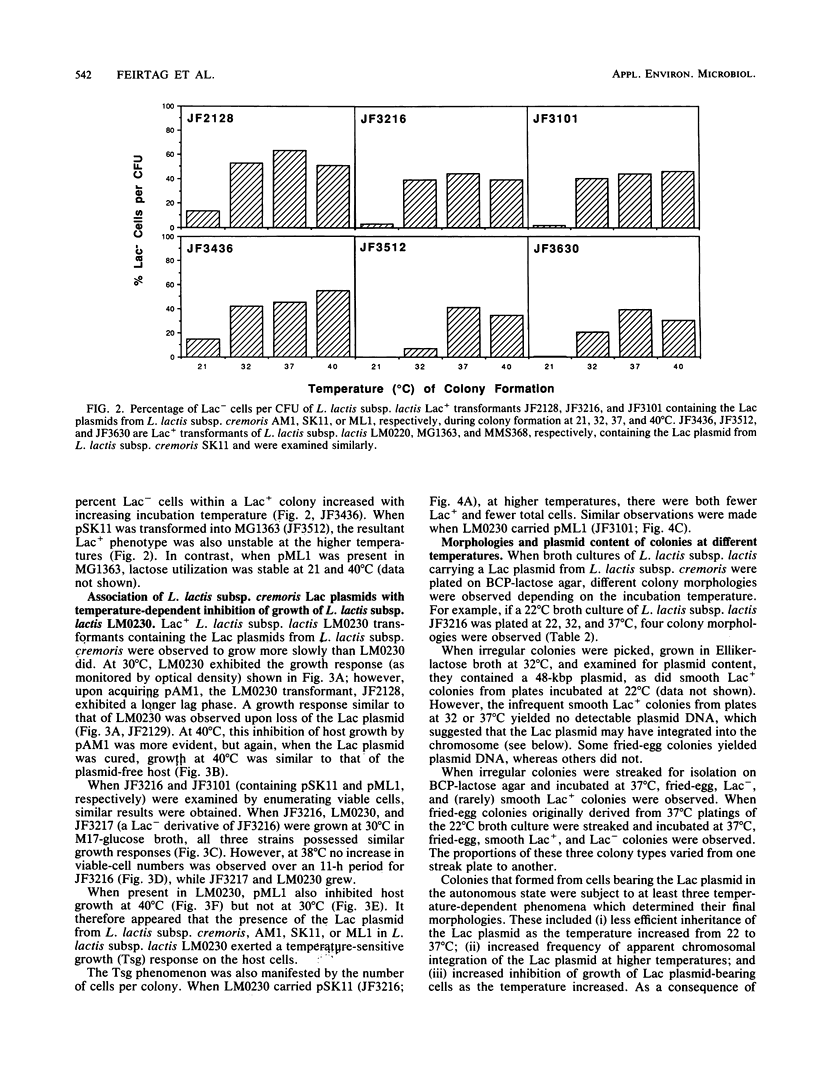
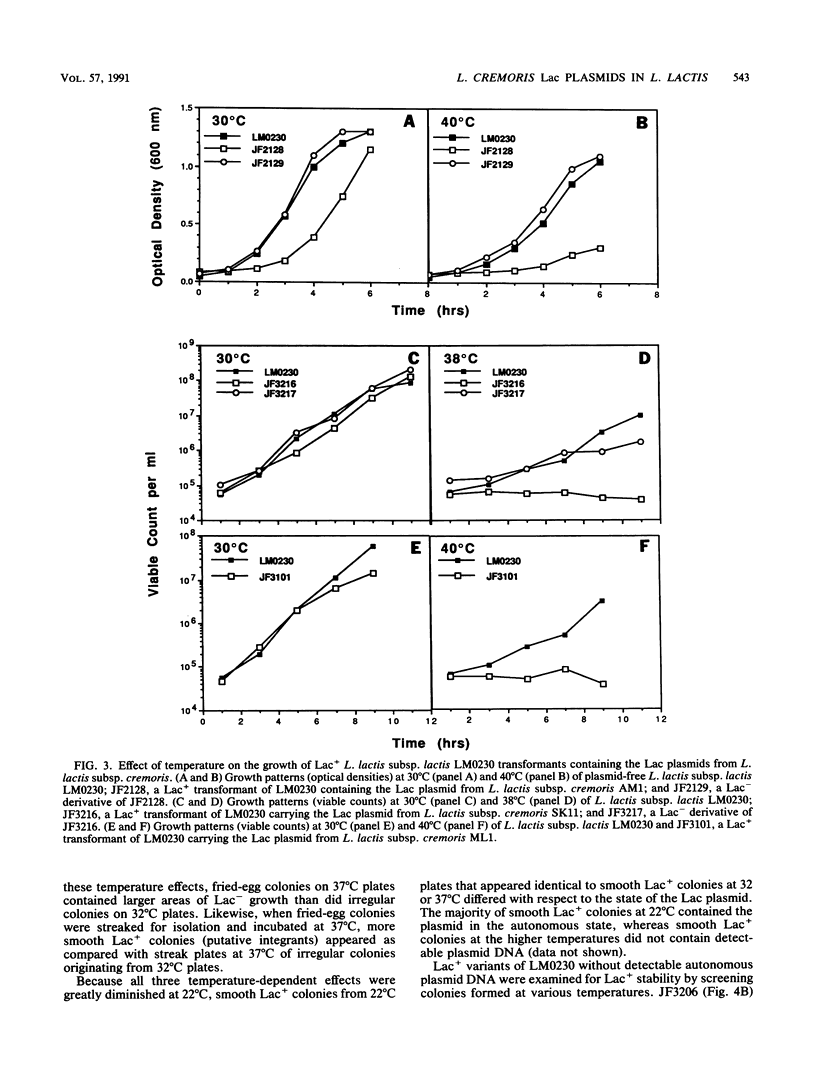
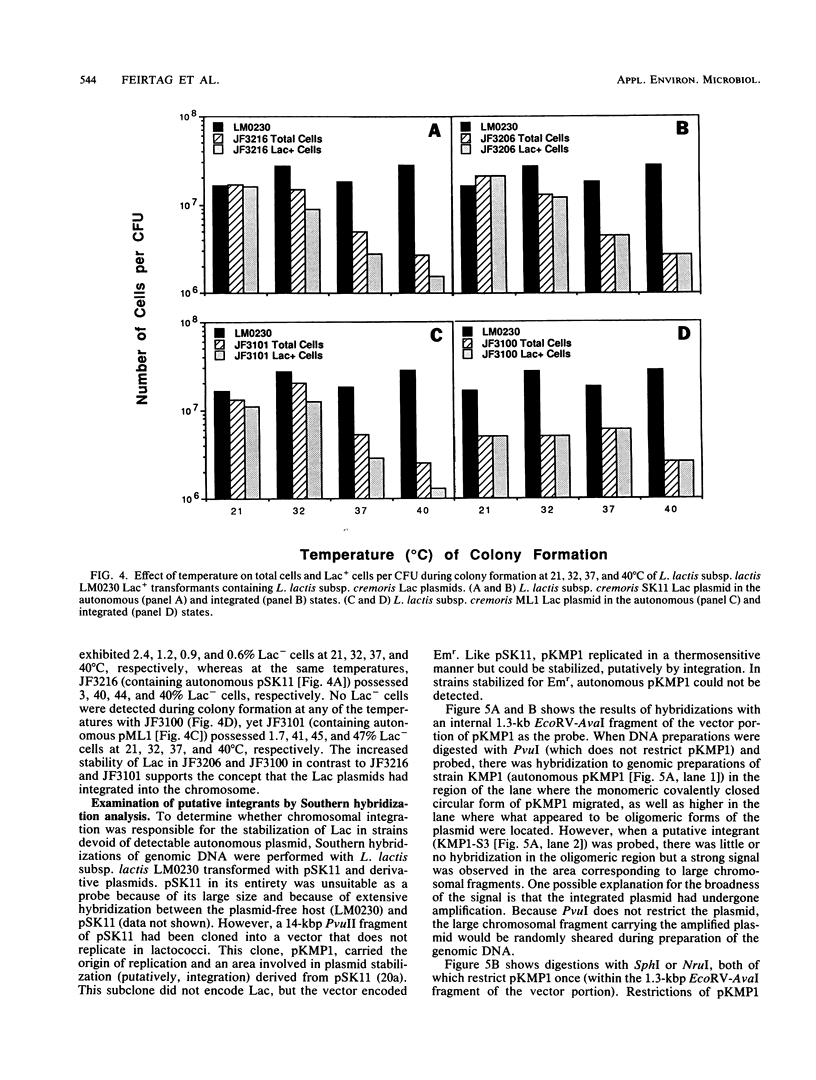
Evidence is presented that lactose-fermenting ability (Lac+) in Lactococcus lactis subsp. cremoris AM1, SK11, and ML1 is associated with plasmid DNA, even though these strains are difficult to cure of Lac plasmids. When the Lac plasmids from these strains were introduced into L. lactis subsp. lactis LM0230, they appeared to replicate in a thermosensitive manner; inheritance of the plasmid was less efficient at 32 to 40 degrees C than at 22 degrees C. The stability of the L. lactis subsp. cremoris Lac plasmids in lactococci appeared to be a combination of both host and plasmid functions. Stabilized variants were isolated by growing the cultures at 32 to 40 degrees C; these variants contained the Lac plasmids integrated into the L. lactis subsp. lactis LM0230 chromosome. In addition, the presence of the L. lactis subsp. cremoris Lac plasmids in L. lactis subsp. lactis resulted in a temperature-sensitive growth response; growth of L. lactis subsp. lactis transformants was significantly inhibited at 38 to 40 degrees C, thereby resembling some L. lactis subsp. cremoris strains with respect to temperature sensitivity of growth.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. G., McKay L. L. Plasmids, loss of lactose metabolism, and appearance of partial and full lactose-fermenting revertants in Streptococcus cremoris B1. J Bacteriol. 1977 Jan;129(1):367–377. doi: 10.1128/jb.129.1.367-377.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. G., McKay L. L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983 Sep;46(3):549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopin M. C., Chopin A., Rouault A., Galleron N. Insertion and amplification of foreign genes in the Lactococcus lactis subsp. lactis chromosome. Appl Environ Microbiol. 1989 Jul;55(7):1769–1774. doi: 10.1128/aem.55.7.1769-1774.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Yagi Y., Dunny G. M., Schultz S. K. Characterization of three plasmid deoxyribonucleic acid molecules in a strain of Streptococcus faecalis: identification of a plasmid determining erythromycin resistance. J Bacteriol. 1974 Jan;117(1):283–289. doi: 10.1128/jb.117.1.283-289.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow V. L., Thomas T. D. Arginine metabolism in lactic streptococci. J Bacteriol. 1982 Jun;150(3):1024–1032. doi: 10.1128/jb.150.3.1024-1032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiJoseph C. G., Bayer M. E., Kaji A. Host cell growth in the presence of the thermosensitive drug resistance factor, Rts1. J Bacteriol. 1973 Jul;115(1):399–410. doi: 10.1128/jb.115.1.399-410.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiJoseph C. G. The thermosensitive lesion in the replication of the drug resistance factor, Rts1. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2515–2519. doi: 10.1073/pnas.71.6.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstathiou J. D., McKay L. L. Inorganic salts resistance associated with a lactose-fermenting plasmid in Streptococcus lactis. J Bacteriol. 1977 Apr;130(1):257–265. doi: 10.1128/jb.130.1.257-265.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froseth B. R., Harlander S. K., McKay L. L. Plasmid-mediated reduced phage sensitivity in Streptococcus lactis KR5. J Dairy Sci. 1988 Feb;71(2):275–284. doi: 10.3168/jds.S0022-0302(88)79555-7. [DOI] [PubMed] [Google Scholar]
- Froseth B. R., Herman R. E., McKay L. L. Cloning of nisin resistance determinant and replication origin on 7.6-kilobase EcoRI fragment of pNP40 from Streptococcus lactis subsp. diacetylactis DRC3. Appl Environ Microbiol. 1988 Aug;54(8):2136–2139. doi: 10.1128/aem.54.8.2136-2139.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasson M. J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983 Apr;154(1):1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempler G. M., McKay L. L. Genetic Evidence for Plasmid-Linked Lactose Metabolism in Streptococcus lactis subsp. diacetylactis. Appl Environ Microbiol. 1979 May;37(5):1041–1043. doi: 10.1128/aem.37.5.1041-1043.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaenhammer T. R., McKay L. L., Baldwin K. A. Improved lysis of group N streptococci for isolation and rapid characterization of plasmid deoxyribonucleic acid. Appl Environ Microbiol. 1978 Mar;35(3):592–600. doi: 10.1128/aem.35.3.592-600.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo J. K., McKay L. L. Plasmid transformation of Streptococcus lactis protoplasts: optimization and use in molecular cloning. Appl Environ Microbiol. 1984 Aug;48(2):252–259. doi: 10.1128/aem.48.2.252-259.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl S. A., Larsen L. D., McKay L. L. Plasmid Profiles of Lactose-Negative and Proteinase-Deficient Mutants of Streptococcus lactis C10, ML(3), and M18. Appl Environ Microbiol. 1979 Jun;37(6):1193–1195. doi: 10.1128/aem.37.6.1193-1195.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen L. D., McKay L. L. Isolation and characterization of plasmid DNA in Streptococcus cremoris. Appl Environ Microbiol. 1978 Dec;36(6):944–952. doi: 10.1128/aem.36.6.944-952.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenhouts K. J., Kok J., Venema G. Campbell-like integration of heterologous plasmid DNA into the chromosome of Lactococcus lactis subsp. lactis. Appl Environ Microbiol. 1989 Feb;55(2):394–400. doi: 10.1128/aem.55.2.394-400.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenhouts K. J., Kok J., Venema G. Stability of Integrated Plasmids in the Chromosome of Lactococcus lactis. Appl Environ Microbiol. 1990 Sep;56(9):2726–2735. doi: 10.1128/aem.56.9.2726-2735.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre D. A., Harlander S. K. Improved electroporation efficiency of intact Lactococcus lactis subsp. lactis cells grown in defined media. Appl Environ Microbiol. 1989 Oct;55(10):2621–2626. doi: 10.1128/aem.55.10.2621-2626.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Efstathiou J. D. Transductional evidence for plasmid linkage of lactose metabolism in streptococcus lactis C2. Appl Environ Microbiol. 1976 Jul;32(1):45–52. doi: 10.1128/aem.32.1.45-52.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Zottola E. A. Loss of lactose metabolism in lactic streptococci. Appl Microbiol. 1972 Jun;23(6):1090–1096. doi: 10.1128/am.23.6.1090-1096.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Cords B. R., Baldwin K. A. Transduction of lactose metabolism in Streptococcus lactis C2. J Bacteriol. 1973 Sep;115(3):810–815. doi: 10.1128/jb.115.3.810-815.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J. T. Mating due to loss of surface exclusion as a cause for thermosensitive growth of bacteria containing the Rtsl plasmid. Mol Gen Genet. 1980;180(3):501–510. doi: 10.1007/BF00268053. [DOI] [PubMed] [Google Scholar]
- Salvi R. J., Ahroon W., Saunders S. S., Arnold S. A. Evoked potentials: computer-automated threshold-tracking procedure using an objective detection criterion. Ear Hear. 1987 Jun;8(3):151–156. [PubMed] [Google Scholar]
- Snook R. J., McKay L. L. Conjugal Transfer of Lactose-Fermenting Ability Among Streptococcus cremoris and Streptococcus lactis Strains. Appl Environ Microbiol. 1981 Nov;42(5):904–911. doi: 10.1128/aem.42.5.904-911.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele J. L., McKay L. L. Conjugal transfer of genetic material by Lactococcus lactis subsp. lactis 11007. Plasmid. 1989 Jul;22(1):32–43. doi: 10.1016/0147-619x(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Terawaki Y., Ishizu K., Horiuchi S., Goto N., Nakaya R. Control of replication and segregation of R plasmid Rts1. J Bacteriol. 1976 Dec;128(3):693–700. doi: 10.1128/jb.128.3.693-700.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Finver S., Yokota T., Bricker J., Kaji A. The region controlling the thermosensitive effect of plasmid Rts1 on host growth is separate from the Rts1 replication region. J Bacteriol. 1981 Apr;146(1):85–92. doi: 10.1128/jb.146.1.85-92.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Kaji A. Replication of thermosensitive Rts1 plasmid deoxyribonucleic acid at the nonpermissive temperature. J Bacteriol. 1977 Oct;132(1):90–99. doi: 10.1128/jb.132.1.90-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]