Abstract
Background
The discovery of diketoacid-containing derivatives as inhibitors of HIV-1 Integrase (IN) (IN inhibitors, IINs) has played a major role in validating this enzyme as an important target for antiretroviral therapy. Since the in vivo efficacy depends on access of these drugs to intracellular sites where HIV-1 replicates, we determined whether the IINs are recognized by the multidrug transporter MDR1-P-glycoprotein (P-gp) thereby reducing their intracellular accumulation. To address the effect of IINs on drug transport, nine quinolonyl diketo acid (DKA) derivatives active on the HIV-1 IN strand transfer (ST) step and with EC50 ranging from 1.83 to >50 μm in cell-based assays were tested for their in vitro interaction with P-gp in the CEM-MDR cell system. IINs were investigated for the inhibition and induction of the P-gp function and expression as well as for multidrug resistance (MDR) reversing ability.
Results
The HIV-1 IINs act as genuine P-gp substrates by inhibiting doxorubicin efflux and inducing P-gp functional conformation changes as evaluated by the modulation of UIC2 mAb epitope. Further, IINs chemosensitize MDR cells to vinblastine and induce P-gp expression in drug sensitive revertants of CEM-MDR cells.
Conclusion
To our knowledge, this is the first demonstration that HIV-1 IINs are P-gp substrates. This biological property may influence the absorption, distribution and elimination of these novels anti HIV-1 compounds.
Background
The emergence of HIV-1 strains resistant to reverse transcriptase and protease inhibitors and the toxicity associated to the chronic use of antiretroviral agents highlights the need to develop antiviral compounds with novel mechanisms of action [1].
The virally encoded integrase (IN) protein is an essential enzyme in the life cycle of the HIV-1 virus and represents an attractive and validated target for the development of antiretroviral agents [2]. Drugs that selectively inhibit this enzyme (IN inhibitors, IINs), when used alone and in combination regimens, have shown potent anti-HIV activity and a good safety profile in phase II clinical trials conducted in treatment-naïve and treatment-experienced HIV+ patients [3-5]
Drug disposition and interaction are important components of the activity and response to antiretroviral drugs. Determinants of drug disposition include the ATP binding cassette (ABC) drug transporter proteins [6]. In particular, considerable attention is now given to understanding the role of the multidrug transporter MDR1-P-glycoprotein (P-gp) in modulating drug bioavailability in cells and tissues [7].
P-gp, which is encoded in humans by the multidrug resistance (MDR) gene 1 (mdr1), is a membrane phosphoglycoprotein that functions as an ATP-dependent drug efflux system for structurally different compounds [8,9]. P-gp was initially studied in the setting of anticancer treatment and was identified as the agent removing a number of drugs from the cells, resulting in what has been termed MDR in tumor cells [10-13]. Concerning HIV-1 infection, it has been recently shown that MDR1-P-gp binds and removes from the drug-treated cells several HIV-1 protease inhibitors (PIs), including the recently approved Atazanavir [8,14-18].
P-gp is naturally present in CD4+ lymphocytes [19-21], one of the main cell targets of HIV-1, and in the endothelial cells lining the small blood capillaries of blood-brain, blood-testis and blood-nerve barriers, preventing the entry of toxic compounds under physiological conditions in potential HIV-1 sanctuary sites in the body [22-24]. The oral bioavailability of drugs and their penetration into the foetus also appear to be hindered by P-gp activity [25]. These findings indicate that P-gp plays an important role in the pharmacokinetic of anti-HIV-1 compounds; however, the inhibition of P-gp induced by different agents or by the combination of anti-HIV-1 drugs themselves may affect the efficacy and penetration of other anti-HIV-1 compounds [8].
On the basis of these considerations, it appears that the effect on MDR1-P-gp expression is an important component of the preclinical evaluation of new antiretroviral compounds, especially IINs, which are among the most promising new anti-HIV-1 agents [26], currently in phase III of clinical development. This study was designed to investigate, by a variety of assays, interactions between IINs and P-gp, potentially influencing their pharmacological activity.
Results and Discussion
Antiviral activity of IINs
Nine in house synthesized IINs [27], selected for their inhibitory activity on the stand transfer (ST) step of HIV-1 integration, were assessed for anti-HIV-1 activity and cytotoxicity on HIV-infected H9 target cells. The results are summarized in Table 1, and show that all tested IINs act as efficient enzyme inhibitors. Three of them (RDS 1974, RDS 1981 and RDS 2022) possessed a relatively low cytotoxicity but exerted a weak antiviral activity (EC50 > 50 μM) in the cell based assay, whereas the RDS 1983, RDS 1984, RDS 1992, RDS 1997 and RDS 2012 exerted a good antiviral activity associated to a relatively low cytotoxicity. In contrast, the good antiviral activity of the RDS 1996 was associated with a relatively high cytoxicity that discouraged its further development as an anti HIV-1 compound.
Table 1.
Inhibition of integration strand transfer, anti-HIV activity and cytotoxicity in the HIV infected H9 cell line of the tested HIV-1 integrase inhibitors.
| Compound (DKA derivatives) | Strand Transfer IC50* (μM) | Anti-HIV activity EC50§ (μM) | Cytotoxicity CC50^ (μM) |
| RDS 1974 | 32 | >50 | >50 |
| RDS 1981 | 0.45 | >50 | >50 |
| RDS 1983 | 0.25 | 5.98 | >50 |
| RDS 1984 | 0.019 | 9.64 | >50 |
| RDS 1992 | 0.70 | 20.5 | >50 |
| RDS 1996 | 0.34 | 24.79 | 2.80 |
| RDS 1997 | 0.012 | 2.44 | >50 |
| RDS 2012 | 0.54 | 1.83 | >50 |
| RDS 2022 | 0.042 | >50 | >50 |
* 50% Inhibitory Concentration; § 50% Effective Concentration; ^ 50% Cytotoxic Concentration
IINs induce a functional P-gp conformation
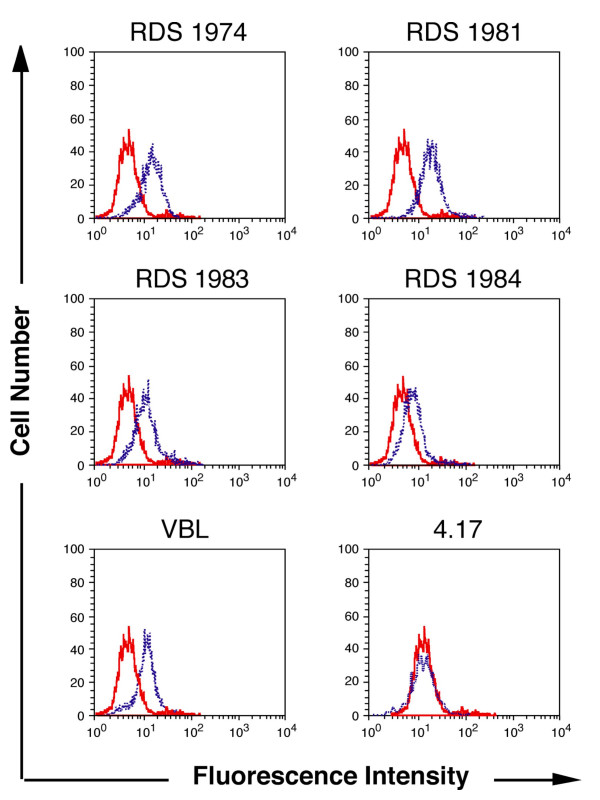
Previous studies demonstrated that the reactivity of the mAb (monoclonal antibody) UIC2 with cells expressing P-gp on their surface is increased at 37°C in the presence of P-gp transport substrates or agents inducing ATP depletion [28-30]. We therefore analyzed the effect of the IINs on UIC2 epitope modulation (Fig 1 and Table 2). To better appreciate this phenomenon the CEM-VBL10 cell line was used as MDR1 cell substrate because of the relatively low number of P-gp binding sites/cell (< 1 × 104) [31-33]. In general, cell lines with a higher level of P-gp require higher concentrations of P-gp substrates for maximal stimulation. As shown in Fig. 1, mAb UIC2 reactivity was increased in the presence of 50 μg/ml each of RDS 1974, RDS 1981, RDS 1983, RDS 1984 and 10 μg/ml vinblastine (VBL), a conventional P-gp substrate, while the binding level of the anti-P-gp mAb, MM4.17 was not modified (Fig. 1). This latter mAb recognizes an extracellular epitope of the human P-gp [30], but does not show the same P-gp ability of mAb UIC2 in intercepting the P-gp modulation during drug transport activity [34]. Importantly, the IINs did not modulate other cell surface antigens such as CD4 (data not shown).
Figure 1.
Functional conformation of P-gp induced by IINs. Fluorescence profile of mAb UIC2 staining on MDR CEM-VBL10 cells incubated in presence of the drug diluent (red histogram), 50 μg/ml of the indicated IINs (blue histogram), or the P-gp substrate vinblastine (10 μg/ml, VBL). MAb MM4.17 staining was carried out in identical conditions in MDR CEM-VBL10 cells incubated with drug diluent (red histogram) or 50 μg/ml of the RDS1974 (blue histogram).
Table 2.
Induction of functional conformation and drug transport inhibition exerted by other IINs in CEM MDR cells.
| Compound | Concentration μg/ml | UIC2 epitope up-modulation (A) | Doxorubicin efflux inhibition (B) |
| RDS 1992 | 25 | NT | + |
| 50 | + | ++ | |
| 100 | ++ | NT | |
| RDS 1996 | 25 | NT | + |
| 50 | NT | ++ | |
| 100 | NT | NT | |
| RDS 1997 | 25 | NT | + |
| 50 | + | ++ | |
| 100 | ++ | NT | |
| RDS 2012 | 25 | NT | + |
| 50 | + | ++ | |
| 100 | ++ | NT | |
| RDS 2022 | 25 | NT | + |
| 50 | + | ++ | |
| 100 | ++ | NT |
A, modulation of UIC2 epitope was studied in CEM-VBL10 cells; B, the effect of IINs on the doxorubicin transport inhibition was analysed in CEM-VBL100 cells; NT, not tested; the + or ++ symbols indicate arbitrary units in measuring and differentiating the level of UIC2 epitope modulation and doxorubicin retention.
Induction of P-gp expression by the IINs in CD4+ CEM cells
Similarly to some of the isozymes involved in drug metabolism, the expression of P-gp is inducible [35]. It is therefore important to assess whether P-gp induction occurs upon exposure to IINs, with potential implications for drug metabolism. To this aim, we investigated whether the treatment with the IINs RDS 1974, RDS 1983, RDS 1984 and RDS 1996, all having different biological and physicochemical properties (Table 1), modulate P-gp expression in the human CD4+ CEM cell system. The parental drug sensitive CEM cell line and the revertant of its derivative CEM-VBL10 MDR cell variant (CEMrev), expressing very low or undetectable amount of P-gp [33], were cultured in the presence of the indicated IINs (10 μg/ml for RDS 1974 and RDS 1996; 25 μg/ml for RDS 1983 and RDS 1984) for 28 days (RDS 1983, RDS 1984 and RDS 1996) or 104 days (RDS 1974). As a control for P-gp modulation, CEMrev cells were exposed to VBL, shown to increase P-gp expression.
Flow cytometry studies (Table 3) showed that, compared with the drug diluent control, exposure to IINs jinduced P-gp over-expression in the CEMrev cell line; the magnitude of this effect depended on treatment duration and relative drug cytotoxicity. In particular, cells that were treated for a longer period of time showed a higher percentage of P-gp positive cells. As expected, VBL exerted a strong induction of P-gp expression in CEMrev cell line. Conversely, no increase in P-gp expression was seen upon exposure of the parental drug sensitive CEM cells to the same IINs. Moreover, IINs treatment and P-gp induction were not associated with modulation of the level of CD4 expression, which was monitored throughout IINs treatment (data not shown). The observation that the IINs RDS 1974, RDS 1983, RDS 1984 and RDS 1996 modulate P-gp expression only in cells having an up-regulation of the mdr1 gene in their in vitro cell culture background [34], suggests that the induction of P-gp by IINs treatment in normal human cells is an unlikely event. Consequently, one may reasonably rule out that these IINs induce P-gp expression in T-lymphocytes of HIV-1 infected patients, leading to reduced antiviral activity of IINs and other P-gp substrates. However, the complexity of the cellular mechanisms involved in the selection of MDR variants has only partially been investigated in this study and it is well known that the MDR phenotype is multifactorial [35]; therefore it cannot be excluded a priori that, under IINs selective pressure, other ABC transporters may be modulated.
Table 3.
Induction of P-gp expression in CEM and CEMrev cell lines exposed to IINs
| Compound | Concentration | Days of culture | % of P-gp expressing cells | |
| CEM | CEMrev | |||
| None | 0 | 1–3 | ||
| RDS 1974 | 10 μg/ml | 104 | 0 | 25–30 |
| RDS 1983 | 25 μg/ml | 28 | 0 | 7–10 |
| RDS 1984 | 25 μg/ml | 28 | 0 | 15–18 |
| RDS 1996 | 10 μg/ml | 28 | 0 | 17–20 |
| Vinblastine | 10 ng/ml | 28 | ND* | 30–35 |
| 104 | ND* | 60–70 | ||
ND, not done
* % of P-gp expressing cells could not be evaluated due to VBL toxicity. Prolonged and stepwise VBL treatment induced high percentage of P-gp expressing cells [31].
P-gp drug efflux is affected by IINs
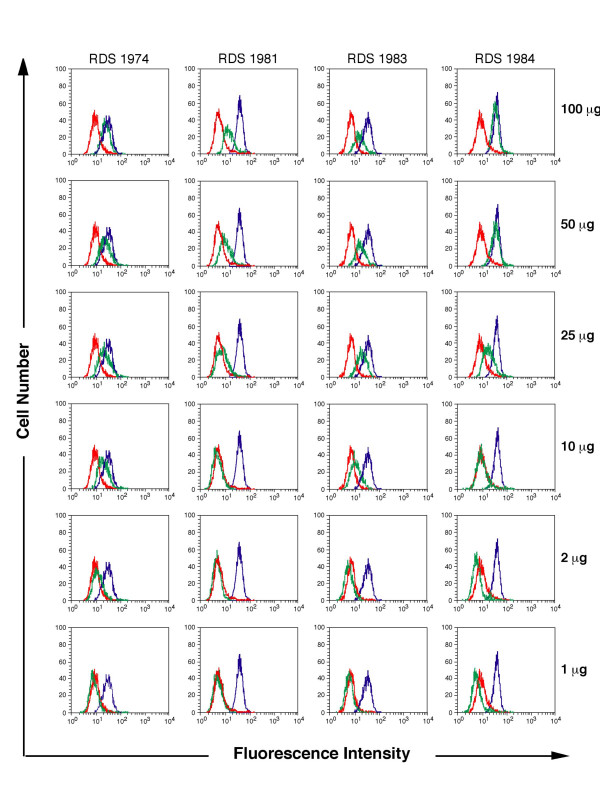
Doxorubucin is a fluorescent substrate for P-gp and incubation of P-gp-positive cells with this drug, followed by washing and further incubation at 37°C, results in a diminished fluorescence profile due to the active drug transport exerted by the efflux system. The presence of P-gp inhibitors such as verapamil during incubation and/or drug extrusion, restores doxorubicin fluorescence. As shown in Fig. 2 the IINs RDS 1974, RDS 1981, RDS 1983 and RDS 1984 are all capable of causing an intracellular accumulation of doxorubicin with a dose dependent effect in CEM-VBL100 MDR cells. The P-gp inhibition exerted by IINs was also investigated in an independent MDR+ cell system. Figure 3 (A) reports the results of confocal microscopy studies showing that KB-V1 P-gp -overexpressing cells at physiological conditions eliminate the dye P-gp substrate doxorubicin from the cells, and a weak or absent fluorescent signal is observed after 1 and 3 hrs of drug extrusion (Fig 3, panels a and d). In contrast, in the presence of verapamil or RDS 1984, the doxorubicin was retained in MDR KB-V1 cells and the drug was detected in cell membranes, nuclei and in marginalized chromatin (Fig 3, panels b-c and e-f). To investigate the potential use of IINs as chemosensitizing MDR agents, their effect in potentiating VBL cytotoxicity in CEM-VBL100 MDR cells was analysed (Fig 3B). Interestingly, all the tested IINs lowered the VBL resistance profile indicating a significant inhibition of P-gp function. In these experiments the IINs exerted a lower MDR reversing ability than verapamil, a drug that has been tested in clinical trials to chemosensitize MDR tumours [36]; nevertheless, in view of their relatively low in vitro cytotoxicity, this class of IINs should be further investigated as potential chemosensitizing compounds for P-gp expressing MDR tumours.
Figure 2.
Drug transport inhibition mediated by IINs. Evaluation of efflux of the dye P-gp substrate doxorubicin in CEM-VBL100 MDR cells. Efflux was monitored in drug-free conditions (red histogram), in the presence of the potent P-gp blocker Verapamil (2.5 μg/ml) (blue histogram) or following incubation with several IINs (RDS1974, RDS1981, RDS1983 and RDS1984) (green histogram) at the indicated concentrations (range 1 μg/ml to 100 μg/ml).
Figure 3.
P-gp inhibition and MDR chemosensitization. In (A), KB-V1 MDR cells were incubated at 37°C for 1 hr with 5 μg/ml doxorubicin alone or in presence of 2.5 μg/ml verapamil or 25 μg/ml RDS 1984. After washing the cells were reincubated again in identical conditions and doxorubicin efflux/retention were analysed in confocal microscopy after 1 h (panel a-c) and 3 hrs (panel d-f). The natural efflux of doxorubicin P-gp mediated is shown in panel a and d, while the doxorubicin retention due to the P-gp drug transport inhibition exerted by verapamil and RDS 1984 is shown in panel b-c (1 h incubation) or e-f (3 hrs incubation). In (B), dose-response cytotoxicity to vinblastine in CEM-VBL100 MDR cells in presence of verapamil (2.5 μg/ml) or 10 μg/ml of the IINs RDS 1974, RDS 1981, RDS 1983 and RDS 1984 is shown. The values (formazan absorbance at 440 nm in ELISA reader) were calculated as % of control cells cultured in presence of IINs only or verapamil. The mean of triplicate measurements is shown; the SD was < 15% of each single value.
Conclusion
IINs are among the most promising agents for the treatment of HIV infection [26], with two of them being in an advanced stage of clinical development [3-5]. To our knowledge, this is the first study showing that IINs are substrates for P-gp. However, we do not know whether this property is shared by all IINs or is restricted to the diketo acid class of IINs tested in this study [27], acting as P-gp substrates by inducing the up-modulation of UIC2 epitope and inhibiting doxorubicin efflux in MDR CEM-VBL100 cells (Table 2 and Fig 2). Concerning the compounds that are under clinical trials, while the MK 0518 [3,4] is a chemically distinct compound, the GS-9137 [5] is a quinolonyl diketoacid derivative comparable to the molecules used in our study. Thus, with good approximation, for the GS-9137 we may hypothesize a similar pattern of response as that observed for the DKA used in the study. Further studies aimed at evaluating the interaction of other clinically significant IINs with the P-gp system will be needed to better address this question.
In our opinion, a successful P-gp modulation may add further interest to this highly promising class of antiretroviral agents and open new perspectives for their clinical use in fields other than HIV infection.
Materials and methods
Integrase Inhibitors and Chemicals
Nine quinolonyl diketoacid derivatives inhibiting the strand transfer step of HIV-1 integration (IC50: 0.042–32 μM) [27] were used in this study. Verapamil (Isoptin) was provided by BASF-Knoll (Milan, Italy), Vinblastine (Velbe) by Eli Lilly (Paris, France) and Doxorubicin by Farmitalia (Nerviano, Italy).
Antiviral activity and cytotoxicity
Anti-HIV activity was measured in the human T lymphoid H9 cells. To this purpose, cells were cultured in RPMI 1640, supplemented with 2 mM L-glutamine, penicillin, streptomycin and 10% fetal bovine serum (FBS), and infected with the HTLVIIIB laboratory strain of HIV-1 virus (100000 TCID50 – Tissue Culture Infective Dose- per 106 PBMC). After two hours of incubation, cells were washed with medium, and cultured at 37°C (5000 cells/well in 96-well microplates) for 3 days in presence of medium and test compounds at concentrations ranging from 50 μM to 0.1 μM. After 3 days p24 antigen concentration in the supernatants was measured by an ELISA assay (Innotest HIV antigen mAb, Innogenetics NV Belgium). Cell viability was determined by the trypan blue exclusion method.
The 50% inhibitory drug concentration (IC50) and the 50% cytotoxic drug concentration (CC50) were calculated by the median effect equation using Calcusyn Version 2.0 program (Biosoft Cambridge)
Cell lines
The multidrug resistant (MDR) variants CEM-VBL10 and CEM-VBL100 cells were isolated by stepwise selection of the parental drug sensitive CCRF-CEM (CEM) in the presence of increasing concentrations of VBL [31,32]. Cells were grown under standard conditions for mammalian cells cultured in suspension. The basic medium (BM) for cell culturing consisted of RPMI-1640 supplemented with 10% foetal calf serum (FCS), L-glutamine (2 mM) penicillin (100 U/mL) and streptomycin (100 U/mL). All these components were purchased from Hyclone (Logan, Utah). Identical BM, culture conditions and trypsin (Hyclone) were used for the adherent MDR variant KB.V1 of the human oral epidermoid carcinoma KB cells [10]. To test the ability of selected IINs (RDS 1974, RDS 1981, RDS 1984 and RDS 1996) to induce de novo expression of P-glycoprotein, the drug-sensitive CEMrev cell line was used; this cell line derives from the CEM-VBL10 MDR cell line, cultured for more than 2 years in VBL-free medium and expresses very low (1–3%) or undetectable amounts of P-gp [32].
MDR efflux assay
CEM-VBL100 cells (1 × 106) were loaded with doxorubicin (10 μg/ml) in 1 ml of BM in the presence of a several IINs (concentrations ranging from 50 μg/ml to 1 mg/ml) or Verapamil (2.5 μg/ml) for 1 h at 37°C. The cells were incubated with doxorubicin (10 μg/ml) only or drug diluent in parallel cultures. At the end of incubation, the cells were washed in serum-free medium and resuspended in BM in the presence of the IINs or Verapamil (drug diluent was added in control samples) for a further 1 h at 37°C. Finally, cells were washed twice with ice-cold PBS/FACS, and analyzed in a flow cytometer (FACScan, Becton Dickinson, San Josè, CA).
Monoclonal antibodies and UIC-2 Shift assay
The anti CD4-FITC mAb was purchased from Vinci Biochem, Firenze, Italy. The mAb UIC2 [30] was kindly provided by Dr. E. Mechetner (Chemicon Inc, Temecula, CA). For determination of P-gp expression, the mAb MM4.17, recognizing an extracellular P-gp epitope on intact/living human MDR cells [28], was also used. Both UIC2 and MM4.17 mAbs were used in a highly purified form.
The UIC2 shift assay was performed under physiological conditions as previously described [29,33]. CEM-VBL10 cells (1 × 106) were resuspended in 1 ml of PBS containing 2% FCS and allowed to equilibrate at 37°C in a water bath for 10 min. The various IINs were added to samples (final concentrations 100 or 50 μg/ml) and incubated for additional 15 min at 37°C with purified UIC2 mAb (final concentration 12.5 μg/ml). VBL (10 μg/ml), which is a well known UIC2 shifting agent, and the drug diluents were used as positive and negative controls, respectively. Cells were then washed twice in ice-cold PBS containing 2% FCS with 0.01 % sodium azide (Shift Stop Buffer, SSB), stained on ice in SSB for additional 15 min with 5 μg/ml of fluorescein -conjugated goat-antimouse antibody (FITC-GAM, Cappel, West Chester, Pa, USA), washed twice with ice cold PBS/FACS and maintained in ice until flow cytometry analysis. The UIC2 shift is the difference between UIC2 binding in the presence versus the absence of the IINs under physiological conditions (37°C).
Flow cytometry and confocal microscopy
For confocal laser-scanning microscopy (CLSM) analyses, KB-V1 adherent cells which express high level of MDR1 P-glycoprotein [10] were grown in WillCo-dishes (WillCo Wells B.V., Amsterdam, The Netherlands) for 24 hours. For P-gp inhibition experiments, the cells were incubated with 5 μg/ml doxorubicin for 1 h at 37°C in presence and absence of the IIN RDS 1984 (25 μg/ml). After washing, the cells were incubated for further 1 and 3 hrs at 37°C in the same above described conditions to allow the efflux/block of doxorubicin. CSLM observations were performed using a Leica TCS 4D apparatus (Leica Lasertechnik GmbH, Heidelberg, Germany), equipped with an argon-krypton laser, 488 nm-dichroic splitter and LP515 long pass filter. Image acquisition and processing were conducted using the SCANware (Leica) and Adobe Photoshop (Adobe Systems Inc., Mountain View, CA) software programs.
MDR reversing
For the evaluation of the MDR reversing ability, CEM-VBL100 cells in exponential phase of growth were collected, extensively washed with warm RPMI-1640 and resuspended at the concentration of 5 × 103 cells/ml in BM alone, or in the presence of the IINs or Verapamil, as appropriated. Then cells were seeded (in triplicate) in 96-wells Costar plates (Costar, Rochester, NY) in which different VBL concentrations were previously added. Within its inhibitory range, the drug decreased growth of all cell lines proportionally to drug concentration. Cell proliferation was determined by adding 10 μg/well of PreMix WST-1 (PreMix WST-1 cell proliferation kit, Vinci Biochem, Firenze, Italy) to the cultures and measuring the absorbance at about 440 nm in a microplate ELISA reader after 4 hrs incubation (48 hrs in total) [37]. The relative cell growth was calculated by applying the formula (En-E0)/(Cn-C0) where E0 and En are the initial and after 48-treatment absorbance values in the drug-containing cultures, and C0 and Cn are the corresponding absorbance values in the untreated control culture. The obtained dose-response profile fulfilled the concentration inhibiting growth by 50% (IC50).
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
CM conceived and planned the biological approach of this study, participated in the design and coordination of the research and drafted the manuscript.
DML conceived and conducted all the cell biological experiments to demonstrate the P-gp substrate activity of the IINs.
MA carried out confocal microscopy studies for the visualization of P-gp mediated activity of the IINs
VA purified and characterized at the biochemical level all the mAbs used in this study and utilized for cell line phenotyping
CR collaborated in the design and synthesis of Integrase Inhibitors
GCM and AM carried out the studies on antiviral activity and cytotoxicity of the IINs in cell based assays.
CA was involved in revising the manuscript critically
DSR conceived and designed the IINs
PL coordinated and supervised the study, interpreted the results and participated in drafting the manuscript
Acknowledgments
Acknowledgements
This work was supported by AIDS grants of Istituto Superiore di Sanità and Italian Ministry of Health and partly by an ISS-NIH research grant.
We wish to thank Mr Marco Sabatini and Mrs Marina Tombesi for graphical and technical support.
Contributor Information
Maurizio Cianfriglia, Email: maurizio.cianfriglia@iss.it.
Maria Luisa Dupuis, Email: marialuisa.dupuis@iss.it.
Agnese Molinari, Email: agnese.molinari@iss.it.
Antonio Verdoliva, Email: verdo@technogen.it.
Roberta Costi, Email: roberta.costi@uniroma1.it.
Clementina Maria Galluzzo, Email: t.galluzzo@iss.it.
Mauro Andreotti, Email: m.andreotti@iss.it.
Andrea Cara, Email: andrea.cara@iss.it.
Roberto Di Santo, Email: roberto.disanto@uniroma1.it.
Lucia Palmisano, Email: lucia.palmisano@iss.it.
References
- Vella S, Palmisano L. The global status of resistance to antiretroviral drugs. Clin Infect Dis. 2005;41:S239–246. doi: 10.1086/430784. [DOI] [PubMed] [Google Scholar]
- Hazuda DJ, Young SD, Guare JP, Anthony NJ, Gomez RP, Wai JS, Vacca JP, Handt L, Motzel SL, Klein HJ, Dornadula G, Danovich RM, Witmer MV, Wilson KA, Tussey L, Schleif WA, Gabryelski LS, Jin L, Miller MD, Casimiro DR, Emini EA, Shiver JW. Integrase inhibitors and cellular immunity suppress retroviral replication in rhesus macaques. Science. 2004;305:528–532. doi: 10.1126/science.1098632. [DOI] [PubMed] [Google Scholar]
- Markowitz M, Morales-Ramirez JO, Nguyen B-Y, Kovacs CM, Steigbigel RT, Cooper DA, Liporace R, Schwartz R, isaacs R, Gilde LR, Wenning L, Zhao J, Teppler H. Antiretroviral activity, pharmacokinetics, and tolerability of MK-0518 a novel inhibitor of HIV-1 integrase, dosed as monotherapy for 10 days in treatment-naúve HIV-1-infected individuals. J Acquir Immune Defic Syndr. 2006;43:509–515. doi: 10.1097/QAI.0b013e31802b4956. [DOI] [PubMed] [Google Scholar]
- Grinsztejn B, Nguyen BY, Katlama C, Gatell J, Lazzarin A, Vittecoq D, Gonzalez C, Chen J, Isaacs R, the Protocol 005 Study Team Potent antiretroviral effect of MK-0518 a novel HIV-1 integrase inhibitor, in patient with triple-class resistant virus. Proceedings of the 13th Conference on Retroviruses and Opportunistic Infection, Denver CO, 5–8 February 2006 Abstract LB 159.
- DeJesus E, Berger D, Markowitz M, Cohen C, Hawkins T, Ruane P, Elion R, Farthing C, Zhong L, Cheng AK, McColl D, Kearney BP, for the 183-0101 Study Team Antiviral activity, pharmacokinetics, and dose response of the HIV integrase inhibitor GS-9137 (JTK-303) in treatment-naive and treatment-experienced patients. J Acquir Immune Defic Syndr. 2006;43:1–5. doi: 10.1097/01.qai.0000233308.82860.2f. [DOI] [PubMed] [Google Scholar]
- Evans WE, McLeod HL. Pharmacogenomics – drug disposition, drug targets, and side effects. N Engl J Med. 2003;348:538–549. doi: 10.1056/NEJMra020526. [DOI] [PubMed] [Google Scholar]
- Schinkel AH. P-Glycoprotein, a gatekeeper in the blood-brain barrier. Adv Drug Deliv Rev. 1999;36:179–194. doi: 10.1016/S0169-409X(98)00085-4. [DOI] [PubMed] [Google Scholar]
- Huisman MT, Smit JW, Schinkel AH. Significance of P-glycoprotein for the pharmacology and clinical use of HIV protease inhibitors. AIDS. 2000;14:237–242. doi: 10.1097/00002030-200002180-00005. [DOI] [PubMed] [Google Scholar]
- Kartner N, Riordan JR, Ling V. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science. 1983;221:1285–1288. doi: 10.1126/science.6137059. [DOI] [PubMed] [Google Scholar]
- Ueda K, Cornwell MM, Gottesman MM, Pastan I, Roninson IB, Ling V, Riordan JR. The mdr1 gene, responsible for multidrug-resistance, codes for P-glycoprotein. Biochem Biophys Res Commun. 1986;141:956–962. doi: 10.1016/S0006-291X(86)80136-X. [DOI] [PubMed] [Google Scholar]
- Germann UA, Pastan I, Gottesman MM. P-glycoproteins: mediators of multidrug resistance. Semin Cell Biol. 1993;4:63–76. doi: 10.1006/scel.1993.1008. [DOI] [PubMed] [Google Scholar]
- Endicott JA, Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Schinkel AH, Borst P. Multidrug resistance mediated by P-glycoproteins. Semin Cancer Biol. 1991;2:213–226. [PubMed] [Google Scholar]
- Kim RB, Fromm MF, Wandel C, Leake B, Wood AJ, Roden DM, Wilkinson GR. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Invest. 1998;101:289–294. doi: 10.1172/JCI1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CG, Gottesman MM, Cardarelli CO, Ramachandra M, Jeang KT, Ambudkar SV, Pastan I, Dey S. HIV-1 protease inhibitors are substrates for the MDR1 multidrug transporter. Biochemistry. 1998;37:3594–3601. doi: 10.1021/bi972709x. [DOI] [PubMed] [Google Scholar]
- Jones K, Hoggard PG, Sales SD, Khoo S, Davey R, Back DJ. Differences in the intracellular accumulation of HIV protease inhibitors in vitro and the effect of active transport. Aids. 2001;15:675–681. doi: 10.1097/00002030-200104130-00002. [DOI] [PubMed] [Google Scholar]
- Washington CB, Duran GE, Man MC, Sikic BI, Blaschke TF. Interaction of anti-HIV protease inhibitors with the multidrug transporter P-glycoprotein (P-gp) in human cultured cells. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:203–209. doi: 10.1097/00042560-199811010-00001. [DOI] [PubMed] [Google Scholar]
- Perloff ES, Duan SX, Skolnik PR, Greenblatt DJ, von Moltke LL. Atazanavir: effects on P-glycoprotein transport and CYP3A metabolism in vitro. Drug Metab Dispos. 2005;33:764–770. doi: 10.1124/dmd.104.002931. [DOI] [PubMed] [Google Scholar]
- Chaudhary PM, Mechetner EB, Roninson IB. Expression and activity of the multidrug resistance P-glycoprotein in human peripheral blood lymphocytes. Blood. 1992;80:2735–2739. [PubMed] [Google Scholar]
- Klimecki WT, Futscher BW, Grogan TM, Dalton WS. P-glycoprotein expression and function in circulating blood cells from normal volunteers. Blood. 1994;83:2451–2458. [PubMed] [Google Scholar]
- Ludescher C, Pall G, Irschick EU, Gastl G. Differential activity of P-glycoprotein in normal blood lymphocyte subsets. Br J Haematol. 1998;101:722–727. doi: 10.1046/j.1365-2141.1998.00751.x. [DOI] [PubMed] [Google Scholar]
- Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci USA. 1987;84:7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordon-Cardo C, O'Brien JP, Boccia J, Casals D, Bertino JR, Melamed MR. Expression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissues. J Histochem Cytochem. 1990;38:1277–1287. doi: 10.1177/38.9.1974900. [DOI] [PubMed] [Google Scholar]
- Hoetelmans RM. Sanctuary sites in HIV-1 infection. Antivir Ther. 1998;3:13–17. [PubMed] [Google Scholar]
- Van Tellingen O. The importance of drug-transporting P-glycoproteins in toxicology. Toxicol Lett. 2001;120:31–41. doi: 10.1016/S0378-4274(01)00304-6. [DOI] [PubMed] [Google Scholar]
- Pommier Y, Johnson AA, Marchand C. Integrase inhibitors to treat HIV/AIDS. Nat Rev Drug Discov. 2005;4:236–248. doi: 10.1038/nrd1660. [DOI] [PubMed] [Google Scholar]
- Di Santo R, Costi R, Roux A, Artico M, Lavecchia A, Marinelli L, Novellino E, Palmisano L, Andreotti M, Amici R, Galluzzo CM, Nencioni L, Palamara AT, Pommier Y, Marchand C. Novel bifunctional quinolonyl diketo acid derivatives as HIV-1 integrase inhibitors: design, synthesis, biological activities, and mechanism of action. J Med Chem. 2006;49:1939–1945. doi: 10.1021/jm0511583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechetner EB, Schott B, Morse BS, Stein WD, Druley T, Davis KA, Tsuruo T, Roninson IB. P-glycoprotein function involves conformational transitions detectable by differential immunoreactivity. Proc Natl Acad Sci USA. 1997;94:12908–12913. doi: 10.1073/pnas.94.24.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy H, Goda K, Arceci R, Cianfriglia M, Mechetner E, Szabo G., Jr P-Glycoprotein conformational changes detected by antibody competition. Eur J Biochem. 2001;268:2416–2420. doi: 10.1046/j.1432-1327.2001.02122.x. [DOI] [PubMed] [Google Scholar]
- Cianfriglia M, Willingham MC, Tombesi M, Scagliotti GV, Frasca G, Chersi A. P-glycoprotein epitope mapping. I. Identification of a linear human-specific epitope in the fourth loop of the P-glycoprotein extracellular domain by MM4.17 murine monoclonal antibody to human multi-drug-resistant cells. Int J Cancer. 1994;56:153–160. doi: 10.1002/ijc.2910560127. [DOI] [PubMed] [Google Scholar]
- Cianfriglia M, Cenciarelli C, Tombesi M, Barca S, Mariani M, Morrone S, Santoni A, Samoggia P, Alessio M, Malavasi F. Murine monoclonal antibody recognizing a 90-kDa cell-surface determinant selectively lost by multi-drug-resistant variants of CEM cells. Int J Cancer. 1990;45:95–103. doi: 10.1002/ijc.2910450118. [DOI] [PubMed] [Google Scholar]
- Cenciarelli C, Currier SJ, Willingham MC, Thiebaut F, Germann UA, Rutherford AV, Gottesman MM, Barca S, Tombesi M, Morrone S, Ramoni C, Cianfriglia M. Characterization by somatic cell genetics of a monoclonal antibody to the MDR1 gene product (P-glycoprotein): determination of P-glycoprotein expression in multi-drug-resistant KB and CEM cell variants. Int J Cancer. 1991;47:533–543. doi: 10.1002/ijc.2910470411. [DOI] [PubMed] [Google Scholar]
- Dupuis ML, Flego M, Molinari A, Cianfriglia M. Saquinavir induces stable and functional expression of the multidrug transporter P-glycoprotein in human CD4 T-lymphoblastoid CEMrev cells. HIV Med. 2003;4:338–345. doi: 10.1046/j.1468-1293.2003.00169.x. [DOI] [PubMed] [Google Scholar]
- Jachez B, Cianfriglia M, Loor F. Modulation of human P-glycoprotein epitope expression by temperature and/or resistance-modulating agents. Anticancer Drugs. 1994;5:655–665. doi: 10.1097/00001813-199412000-00008. [DOI] [PubMed] [Google Scholar]
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2:48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]