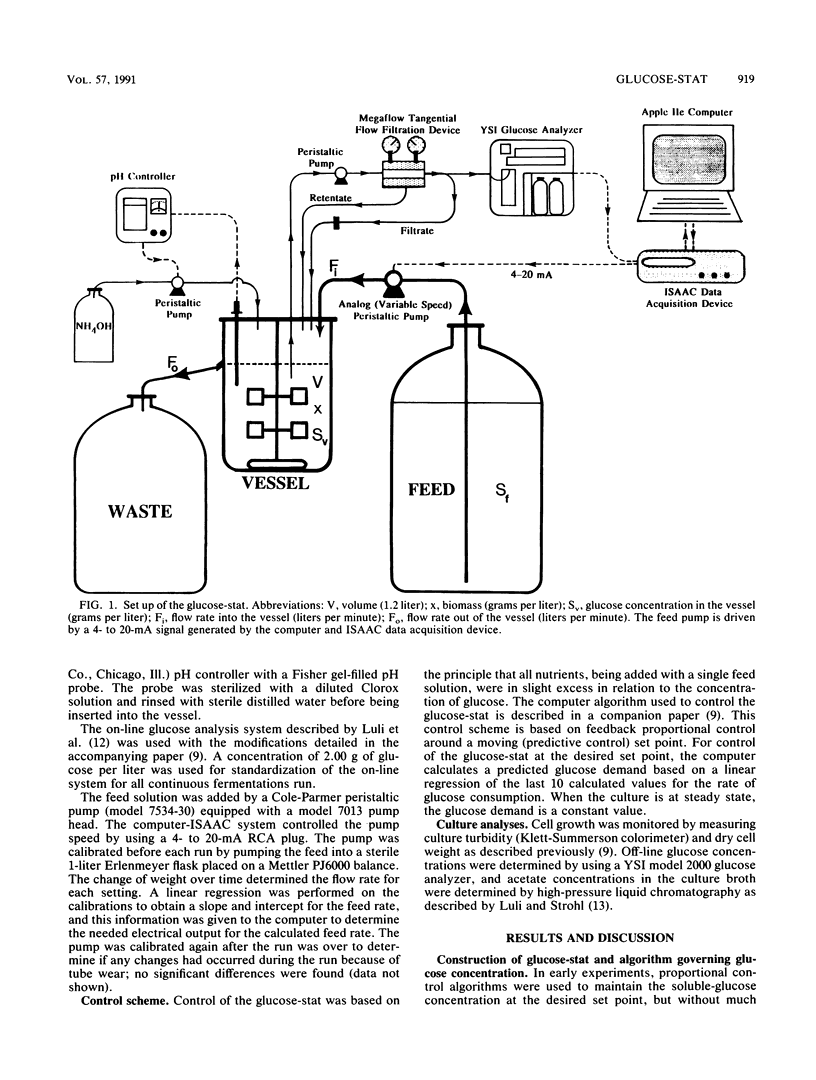
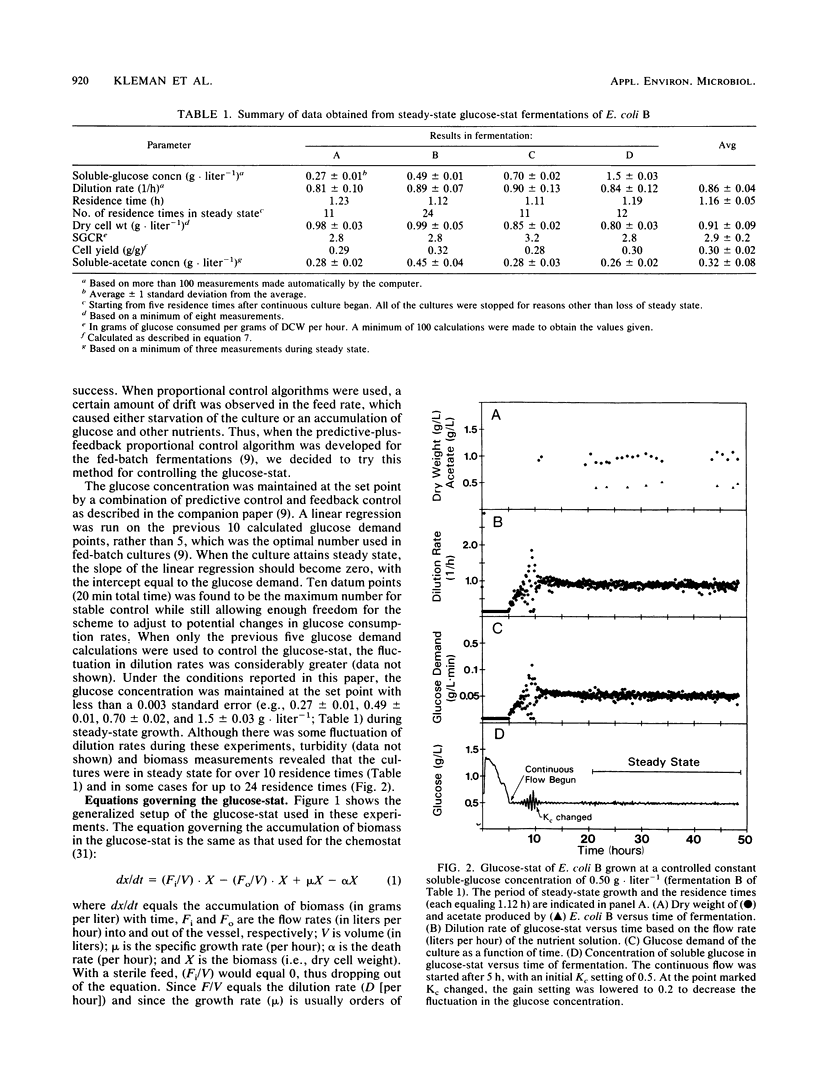
Abstract
A predictive and feedback proportional control algorithm, developed for fed-batch fermentations and described in a companion paper (G. L. Kleman, J. J. Chalmers, G. W. Luli, and W. R. Strohl, Appl. Environ. Microbiol. 57:910-917, 1991), was used in this work to control a continuous culture on the basis of the soluble-glucose concentration (called the glucose-stat). This glucose-controlled continuous-culture system was found to reach and maintain steady state for 11 to 24 residence times when four different background glucose concentrations (0.27, 0.50, 0.7, and 1.5 g/liter) were used. The predictive-plus-feedback control system yielded very tight control of the continuous nutristat cultures; glucose concentrations were maintained at the set points with less than 0.003 standard error. Acetate production by Escherichia coli B in glucose-stats was found not to be correlated with the level of steady-state soluble-glucose concentration.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Doelle H. W., Hollywood N., Westwood A. W. Effect of glucose concentration on a number of enzymes involved in the aerobic and anaerobic utilization of glucose in turbidostat-cultures of Escherichia coli. Microbios. 1974 Mar-Apr;9(36):221–232. [PubMed] [Google Scholar]
- Hollywood N., Doelle H. W. Effect of specific growth rate and glucose concentration on growth and glucose metabolism of Escherichia coli K-12. Microbios. 1976;17(67):23–33. [PubMed] [Google Scholar]
- Kleman G. L., Chalmers J. J., Luli G. W., Strohl W. R. A predictive and feedback control algorithm maintains a constant glucose concentration in fed-batch fermentations. Appl Environ Microbiol. 1991 Apr;57(4):910–917. doi: 10.1128/aem.57.4.910-917.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luli G. W., Strohl W. R. Comparison of growth, acetate production, and acetate inhibition of Escherichia coli strains in batch and fed-batch fermentations. Appl Environ Microbiol. 1990 Apr;56(4):1004–1011. doi: 10.1128/aem.56.4.1004-1011.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald H. L., Neway J. O. Effects of medium quality on the expression of human interleukin-2 at high cell density in fermentor cultures of Escherichia coli K-12. Appl Environ Microbiol. 1990 Mar;56(3):640–645. doi: 10.1128/aem.56.3.640-645.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesenberg D., Bergter F. Mixed culture kinetics of stringent and relaxed Escherichia coli cells in glucose-limited chemostat. Z Allg Mikrobiol. 1984;24(2):113–117. doi: 10.1002/jobm.3630240210. [DOI] [PubMed] [Google Scholar]
- Titus J. A., Luli G. W., Dekleva M. L., Strohl W. R. Application of a microcomputer-based system to control and monitor bacterial growth. Appl Environ Microbiol. 1984 Feb;47(2):239–244. doi: 10.1128/aem.47.2.239-244.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


