Abstract
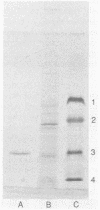
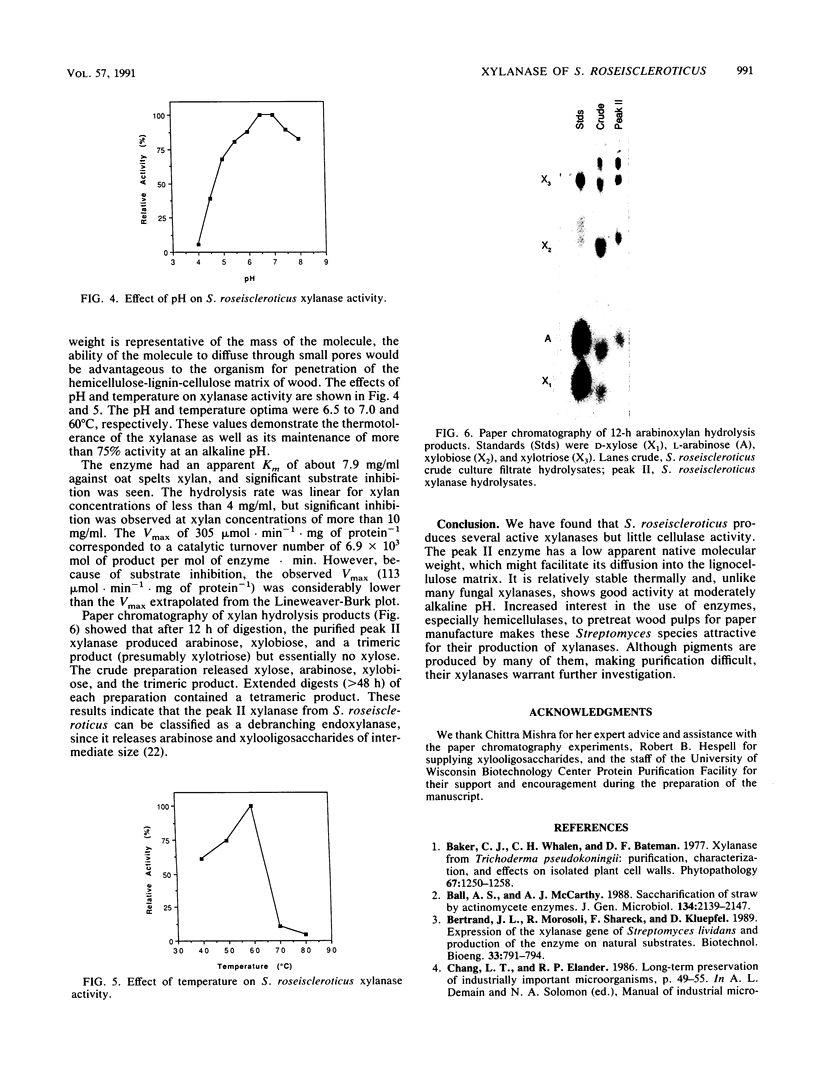
Twelve species of Streptomyces that formerly belonged to the genus Chainia were screened for the production of xylanase and cellulase. One species, Streptomyces roseiscleroticus (Chainia rosea) NRRL B-11019, produced up to 16.2 IU of xylanase per ml in 48 h. A xylanase from S. roseiscleroticus was purified and characterized. The enzyme was a debranching β-(1-4)-endoxylanase showing high activity on xylan but essentially no activity against acid-swollen (Walseth) cellulose. It had a very low apparent molecular weight of 5,500 by native gel filtration, but its denatured molecular weight was 22,600 by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. It had an isoelectric point of 9.5. The pH and temperature optima for hydrolysis of arabinoxylan were 6.5 to 7.0 and 60°C, respectively, and more than 75% of the optimum enzyme activity was retained at pH 8.0. The xylanase had a Km of 7.9 mg/ml and an apparent Vmax of 305 μmol · min-1 · mg of protein-1. The hydrolysis rate was linear for xylan concentrations of less than 4 mg/ml, but significant inhibition was observed at xylan concentrations of more than 10 mg/ml. The predominant products of arabinoxylan hydrolysis included arabinose, xylobiose, and xylotriose.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dekker R. F., Richards G. N. Purification, properties, and mode of action of hemicellulase I produced by Ceratocystis paradoxa. Carbohydr Res. 1975 Jan;39(1):97–114. doi: 10.1016/s0008-6215(00)82642-7. [DOI] [PubMed] [Google Scholar]
- Morosoli R., Bertrand J. L., Mondou F., Shareck F., Kluepfel D. Purification and properties of a xylanase from Streptomyces lividans. Biochem J. 1986 Nov 1;239(3):587–592. doi: 10.1042/bj2390587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. R., Trask J. D. Occurrence and Recovery of the Virus of Infantile Paralysis from Sewage. Am J Public Health Nations Health. 1942 Mar;32(3):235–239. doi: 10.2105/ajph.32.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly P. J. Xylanases: structure and function. Basic Life Sci. 1981;18:111–129. doi: 10.1007/978-1-4684-3980-9_8. [DOI] [PubMed] [Google Scholar]
- Robyt J. F., Whelan W. J. Reducing value methods for maltodextrins. I. Chain-length dependence of alkaline 3,5-dinitrosalicylate and chain-length independence of alkaline copper. Anal Biochem. 1972 Feb;45(2):510–516. doi: 10.1016/0003-2697(72)90213-8. [DOI] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]