Abstract
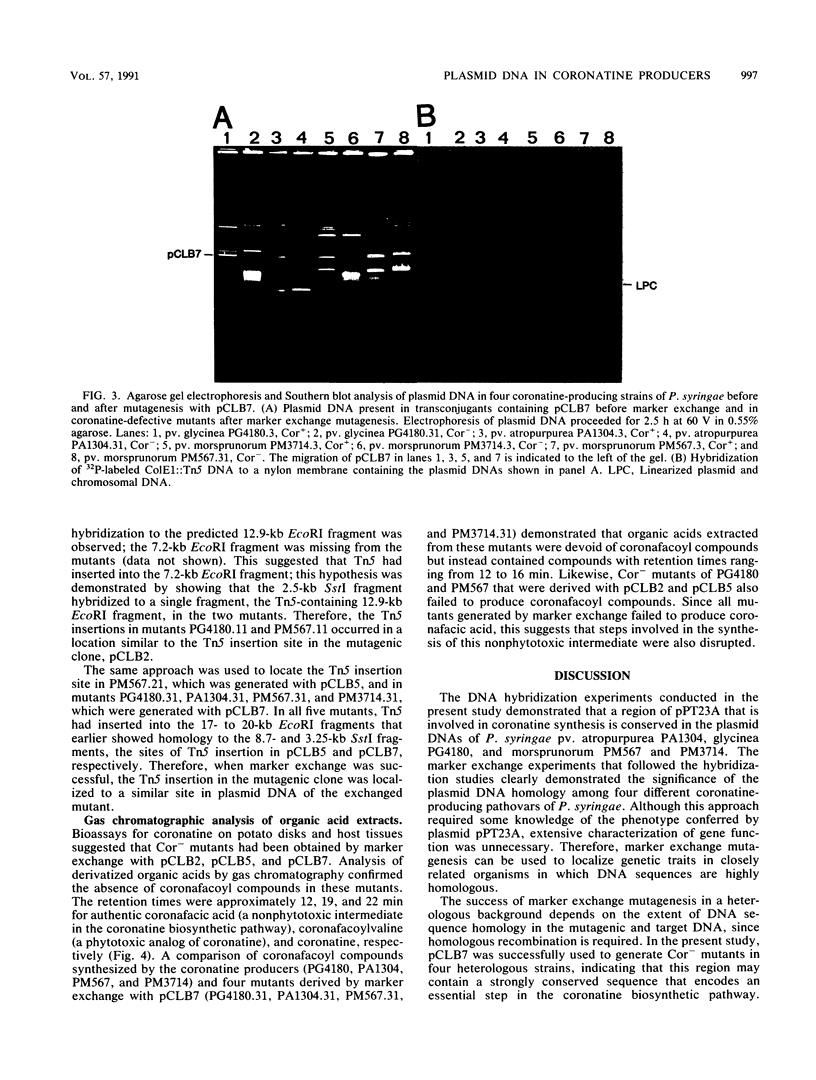
In Pseudomonas syringae pv. tomato PT23.2, plasmid pPT23A (101 kb) is involved in synthesis of the phytotoxin coronatine (C. L. Bender, D. K. Malvick, and R. E. Mitchell, J. Bacteriol. 171:807-812, 1989). The physical characterization of mutations that abolished coronatine production indicated that at least 30 kb of pPT23A DNA are required for toxin synthesis. In the present study, 32P-labeled DNA fragments from the 30-kb region of pPT23A hybridized to plasmid DNAs from several coronatine-producing pathovars of P. syringae under conditions of high stringency. These experiments indicated that this region of pPT23A was strongly conserved in large plasmids (90 to 105 kb) that reside in P. syringae pv. atropurpurea, glycinea, and morsprunorum. The functional significance of the observed homology was demonstrated in marker-exchange experiments in which Tn5-inactivated sequences from the 30-kb region of pPT23A were used to mutate coronatine synthesis genes in the three heterologous pathovars. Physical characterization of the Tn5 insertions generated by marker exchange indicated that genes controlling coronatine synthesis in P. syringae pv. atropurpurea 1304, glycinea 4180, and morsprunorum 567 and 3714 were located on the large indigenous plasmids where homology was originally detected. Therefore, coronatine biosynthesis genes are strongly conserved in the plasmid DNAs of four producing pathovars, despite their disparate origins (California, Japan, New Zealand, Great Britain, and Italy).
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender C. L., Cooksey D. A. Indigenous plasmids in Pseudomonas syringae pv. tomato: conjugative transfer and role in copper resistance. J Bacteriol. 1986 Feb;165(2):534–541. doi: 10.1128/jb.165.2.534-541.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender C. L., Cooksey D. A. Molecular cloning of copper resistance genes from Pseudomonas syringae pv. tomato. J Bacteriol. 1987 Feb;169(2):470–474. doi: 10.1128/jb.169.2.470-474.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender C. L., Malvick D. K., Conway K. E., George S., Pratt P. Characterization of pXV10A, a Copper Resistance Plasmid in Xanthomonas campestris pv. vesicatoria. Appl Environ Microbiol. 1990 Jan;56(1):170–175. doi: 10.1128/aem.56.1.170-175.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender C. L., Malvick D. K., Mitchell R. E. Plasmid-mediated production of the phytotoxin coronatine in Pseudomonas syringae pv. tomato. J Bacteriol. 1989 Feb;171(2):807–812. doi: 10.1128/jb.171.2.807-812.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse G. F., Frischauf A., Lehrach H. An integrated and simplified approach to cloning into plasmids and single-stranded phages. Methods Enzymol. 1983;101:78–89. doi: 10.1016/0076-6879(83)01006-x. [DOI] [PubMed] [Google Scholar]
- Eberhard W. G. Why do bacterial plasmids carry some genes and not others? Plasmid. 1989 May;21(3):167–174. doi: 10.1016/0147-619x(89)90040-1. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Figurski D. H., Helinski D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Keen N. T., Tamaki S., Kobayashi D., Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988 Oct 15;70(1):191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- Staskawicz B., Dahlbeck D., Keen N., Napoli C. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J Bacteriol. 1987 Dec;169(12):5789–5794. doi: 10.1128/jb.169.12.5789-5794.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G. W., Gross D. C. Physical and functional analyses of the syrA and syrB genes involved in syringomycin production by Pseudomonas syringae pv. syringae. J Bacteriol. 1988 Dec;170(12):5680–5688. doi: 10.1128/jb.170.12.5680-5688.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]




