Abstract
Glucosylation of RhoA, Rac1, and Cdc42 by Clostridium difficile toxin B from strain VPI 10463 (TcdB) results in actin reorganization (cytopathic effect) and apoptosis (cytotoxic effect). Toxin B from variant C. difficile strain 1470 serotype F (TcdBF) differs from TcdB with regard to substrate proteins, as it glucosylates Rac1 and R-Ras but not RhoA and Cdc42. In this study, we addressed the question of whether the cellular effects of the toxins depend on their protein substrate specificity. Rat basophilic leukemia (RBL) cells were synchronized using the thymidine double-block technique. We show that cells were most sensitive to the cytotoxic effect of TcdB in S phase, as analyzed in terms of phosphatidyl serine externalization, fragmentation of nuclei, and activation of caspase-3; in contrast, TcdBF induced only a marginal cytotoxic effect, suggesting that inactivation of RhoA (but not of Rac1) was required for the cytotoxic effect. The glucosylation of Rac1 was correlated to the cytopathic effect of either toxin, suggesting a close connection of the two effects. The cytotoxic effect of TcdB was executed by caspase-3, as it was responsive to inhibition by acetyl-Asp-Met-Gln-Asp-aldehyde (Ac-DMQD-CHO), an inhibitor of caspase-3. The viability of TcdB-treated RBL cells was reduced, whereas the viability of TcdBF-treated cells was unchanged, further confirming that inactivation of RhoA is required for the cytotoxic effect. In conclusion, the protein substrate specificity of the glucosylating toxins determines their biological activity.
Pathogenic strains of Clostridium difficile cause intestinal infections ranging from mild self-curing diarrhea to the severe form, pseudomembranous colitis (24, 44). These strains produce two toxins, toxin A (TcdA) and toxin B (TcdB) (toxinotype A+ B+). Both toxins are glucosyltransferases that covalently modify Rho, Rac, and Cdc42 (25). These Rho proteins are involved in the control of actin dynamics, cell cycle progression, gene transcription, and vesicle trafficking (10, 39, 45). The glucosylation site (Thr-37 in RhoA, Thr-35 in Rac1 and Cdc42) is located within the effector region of Rho proteins, resulting in impairment of effector and regulator coupling (16, 41, 43). Impaired Rho signaling results in reorganization of the actin cytoskeleton (cytopathic effect) and in cell death (cytotoxic effect) (9, 13).
Most work on the cytotoxic effect has been performed on TcdA (5, 6, 26, 31). However, the cytotoxic effect of TcdB may also be important for C. difficile-associated disease; e.g., activation of caspase-3 and caspase-9 has been shown to be involved in the cytotoxic effect of TcdB (19, 27, 29, 38).
Some C. difficile strains are described as producing solely toxin B and are therefore classified as variant strains (toxinotype A− B+) (40). Although toxin B from variant Clostridium difficile strain 1470 serotype F (TcdBF) exhibits an identity of about 93% with TcdB from reference strain VPI 10463 at the amino acid level, TcdBF differs from TcdB with regard to protein substrate specificity: TcdBF glucosylates Rac and R-Ras but neither Rho nor Cdc42 (7, 8).
In this study, we show that rat basophilic leukemia (RBL) cells are most sensitive to the cytotoxic effect of the Rho/Rac/Cdc42-glucosylating TcdB in S phase. In contrast, variant TcdBF, which inactivates Rac and R-Ras, induces only a marginal cytotoxic effect, strongly suggesting that initiation of the cytotoxic effect depends on the substrate specificity of the glucosylating toxins.
MATERIALS AND METHODS
Caspase inhibitor I {z-Val-Ala-Asp(OMe)-CH2F [Z-VAD(OMe)-FMK]}, caspase-3 inhibitor (acetyl-Asp-Met-Gln-Asp-aldehyde [Ac-DMQD-CHO]), latrunculin B, and staurosporine were from Calbiochem. WST-1 {4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate} was obtained from Roche. Anti-Rac1 (clone 102) and anti-Cdc42 (clone 44) were from Transduction Laboratories. Anti-Rac1 (clone 23A8) was purchased from Upstate Technologies. Anti-caspase-3 was from Acris. Anti-beta-actin (clone AC-40) was obtained from Sigma. Horseradish peroxidase-conjugated secondary antibodies to mouse and rabbit immunoglobulin were from Rockland. Anti-RhoA (clone 26C4) was from Santa Cruz. Anti-RhoB (BL927) was obtained from Bethyl Laboratories.
Toxin purification.
Toxin B from Clostridium difficile strain VPI 10463 (TcdB) and variant toxin B from C. difficile strain 1470 were purified as described previously (17). In brief, a dialysis bag containing 900 ml of 0.9% NaCl in a total volume of 4 liters of brain heart infusion (Difco) was inoculated with 100 ml of an overnight culture of C. difficile, and the culture was grown under microaerophilic conditions at 37°C for 72 h. Proteins were precipitated from the culture supernatant by ammonium sulfate at 70% saturation. The precipitates were dialyzed against Tris-HCl, pH 7.5, buffer overnight and loaded onto a MonoQ column (Amersham Biosciences). The toxins were eluted with 50 mM Tris-HCl, pH 7.5, buffer containing 500 mM NaCl and subsequently dialyzed against buffer (50 mM Tris-HCl [pH 7.5], 15 mM NaCl).
Cell culture and synchronization.
RBL cells are sensitive to glucosylating toxins and are a well-established cell line for studying apoptotic cell death; furthermore, RBL cells can be synchronized using the thymidine double-block technique (20, 35, 36, 42). RBL cells were cultured in minimum essential medium supplemented with 15% heat-inactivated fetal calf serum, 100 μg/ml penicillin, 100 U/ml streptomycin, and 1 mM sodium pyruvate. NIH 3T3 fibroblasts and macrophage-like J774A.1 cells (a kind gift of Peter Sebo and Jana Kamanova, Prague, Czech Republic) were cultivated in Dulbecco's modified essential medium (Biochrom; with 10% FCS, 100 μg/ml penicillin, 100 U/ml streptomycin, and 1 mM sodium pyruvate). Cells were maintained in 5% CO2 at 37°C. Cell synchronization was performed by the thymidine double-block technique (20). Exponentially dividing cells were incubated in medium containing 2 mM 2′-deoxythymidine for 19 h followed by a growth phase of 9 h in thymidine-free medium. The cells were synchronized at the next G1/S boundary by incubation in medium containing 2 mM 2′-deoxythymidine for an additional 16 h. After removal of the second block, cells were incubated for 1 h in thymidine-free medium and then treated with the toxins or drugs as indicated in Fig. 2 to 4. Cells were harvested as indicated and analyzed for apoptosis.
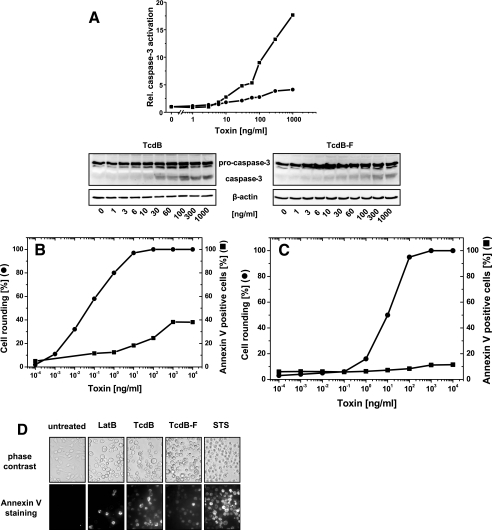
FIG. 2.
Cytotoxic effect in nonsynchronized RBL cells. (A) Activation of caspase-3 by TcdB. RBL cells were exposed to increasing concentrations of TcdB (▪) or TcdBF (•) for 3 h. The cellular levels of procaspase-3 and caspase-3 were determined by Western blot analysis. The signal intensity was normalized to the intensity of the beta-actin signal. (B) Cytotoxic effect of TcdB. RBL cells were exposed to increasing concentrations of TcdB for 24 h. The cytopathic effect was determined as the percent rounded cells. Annexin V-positive cells were stained with annexin V-Alexa Fluor 488 and visualized by fluorescence microscopy. (C) Cytotoxic effect of TcdB. RBL cells were exposed to increasing concentrations of TcdBF for 24 h. The cytopathic effect was determined as the percent rounded cells. Annexin V-positive cells were stained with annexin V-Alexa Fluor 488 and visualized by fluorescence microscopy. (D) Cytotoxic effect in nonsynchronized RBL cells. RBL cells were exposed to latrunculin B (2.5 μM), staurosporine (STS) (0.2 μM), TcdB (0.1 μg/ml), or TcdBF (1 μg/ml) or left untreated for 24 h. Phosphatidylserine exposure was visualized by annexin V staining.
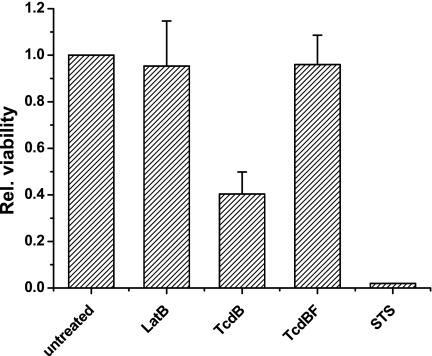
FIG. 4.
Viability of toxin-treated RBL cells. Synchronized RBL cells were exposed to latrunculin B (2.5 μM), staurosporine (0.2 μM), TcdB (0.1 μg/ml), or TcdBF (1 μg/ml) for 24 h. Viability was assessed by the WST-1 test. The viability of untreated cells was set at 1.0. Results are means of three independent experiments plus standard deviations.
Analysis of apoptosis.
After toxin treatment, annexin V labeled with Alexa Fluor 488 (Cambrex) and 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (0.1 μg/ml) was added directly into the medium, according to the manufacturer's instructions. After incubation at 37°C for 15 min, cells were analyzed by fluorescence microscopy using a Zeiss Axiovert 200 M (Alexa Fluor 448: excitation, 470 nm; emission, 515 nm; DAPI: excitation 365 nm, emission 420 nm). Among the annexin V-positive RBL cells, some cells were also DAPI positive, indicating a loss of membrane integrity; the nuclei of these cells, however, were fragmented, indicating that these cells were apoptotic prior to the loss of membrane integrity (secondary necrosis) (data not shown).
To determine in situ chromatin condensation, cells were washed with phosphate-buffered saline and subsequently fixed and permeabilized in a buffer containing 3.7% formaldehyde and 0.1% (wt/vol) Triton X-100 at room temperature for 1 h. Then, a 0.5-μg/ml solution of DAPI in Tris-buffered saline supplemented with 0.5% (wt/vol) Tween 20 was added, and the cells were incubated at 37°C for 1 h. Cells were analyzed by fluorescence microscopy using a Zeiss Axiovert 200 M (excitation, 365 nm; emission, 420 nm).
Western blot analysis.
After toxin treatment, RBL cells were washed and scraped into Laemmli sample buffer. The obtained suspension was sonicated on ice and incubated at 95°C for 10 min. Complete lysates were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and subsequently transferred onto nitrocellulose membranes by a tank blot system. The membranes were blocked with 5% (wt/vol) nonfat dried milk in Tris-buffered saline supplemented with 0.1% Tween 20 for 60 min. Incubation with primary antibody was conducted over night at 4°C, treatment with the secondary antibody at 22°C for 1 h. For the chemiluminescence reaction, ECL Femto (Pierce) was used.
DNA flow cytometry.
DNA content was determined by flow cytometry. Cells (106) were washed in phosphate-buffered saline and fixed in 70% ethanol on ice for 30 min. The cells were then centrifuged at 400 × g for 5 min. Subsequently, they were stained with propidium iodide (150 μg/ml in phosphate-buffered saline supplemented with 1% bovine serum albumin and 1% Triton X-100). RNA was removed by incubation with 0.1 mg/ml RNase A at 24°C for 30 min. The stained cells were analyzed using a FACScan flow cytometer (Becton Dickinson). A sequence of single-parameter DNA histograms was analyzed to estimate the proportions of cells in each phase.
WST-1 viability assay.
Cell viability was determined utilizing WST-1 (Roche) according to the manufacturer's instructions. Briefly, subconfluent RBL cells inoculated in microtiter plates (96 well, tissue culture grade, flat bottom) were exposed to the toxins or drugs as indicated in Fig. 4 for 24 h. Subsequently, WST-1 was added to each well. WST-1 is a tetrazolium salt that is reduced to formazan by mitochondrial dehydrogenases. Formazan dye was quantitated using a scanning multiwell spectrophotometer at 450 nm.
Data analysis.
The 50% effective concentrations (EC50s) for the glucosylation of Rac1 and the cytopathic effect were obtained by fitting experimental data to the Hill equation (28). The quality of the fit was assessed by comparison of the parameter standard errors, correlation coefficients, and 95% confidence intervals.
RESULTS
Activity of toxin B and variant toxin B in RBL cells.
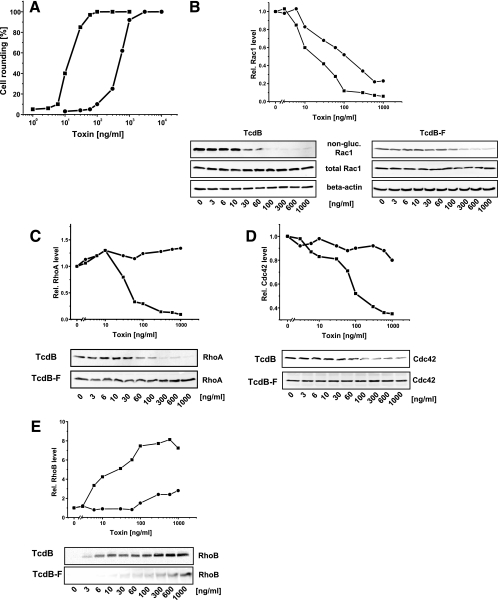
Glucosylation of Rho and Ras proteins by toxin B (TcdB) and variant toxin B (TcdBF) induces actin reorganization in cultured cells (cytopathic effect) (7, 8). RBL cells were treated with increasing concentrations of either TcdB or TcdBF for 4 h. The cytopathic effect was recorded as the number of rounded cells per total cells (Fig. 1A). The concentration-cytopathic effect curve was sigmoid for both toxins, with TcdB being 1 order of magnitude more potent than TcdBF (Fig. 1A).
FIG. 1.
Cellular activity of TcdB and TcdBF in RBL cells. (A) Cytopathic effect of TcdB and TcdBF. RBL cells were exposed to TcdB (▪) or TcdBF (•) for 3 h. The cytopathic effect was determined as percent rounded cells. (B to E) Influence of the toxins on the cellular levels of Rho proteins. RBL cells were exposed to TcdB (▪) or TcdBF (•) for 3 h. The cellular levels of Rho proteins were determined by Western blotting with the indicated antibodies. The signal intensity was normalized to the intensity of the corresponding beta-actin signal.
To confirm that the cytopathic effect was based on the glucosylation of cellular Rho proteins, glucosylation of Rac1 was utilized as a marker of the cellular activity of the toxins (17). The glucosylation of Rac1 was detected as an apparent decrease of the cellular Rac1 level by applying anti-Rac1 (monoclonal antibody [MAb] 102), an antibody that reacts exclusively to nonglucosylated Rac1 (17). The level of Rac1 apparently decreased in response to increasing concentrations of either toxin, showing the glucosylation of cellular Rac1 (Fig. 1B). The cellular level of Rac1 was further analyzed by anti-Rac1 (MAb 23A8), an antibody that recognizes both glucosylated and nonglucosylated Rac1 (17). The level of total Rac1 was indeed unchanged in toxin-treated cells, confirming that the apparently decreasing level of Rac1 revealed by anti-Rac1 (MAb 102) was due to glucosylation and not degradation of Rac1 (Fig. 1B) (17). The level of beta-actin was not altered by toxin treatment and was therefore used as an internal standard for identical protein content (Fig. 1B to E). The increasing glucosylation of Rac1 correlated closely with the increasing cytopathic effect, supporting our recently published suggestion that glucosylated Rac1 is a marker protein of the cellular activity of the glucosylating toxins (17). The sigmoid concentration-effect curves were fitted to the Hill equation, and the correlation coefficient was 0.95 or better. The EC50s of TcdB and TcdBF for the cytopathic effect were 13 ng/ml and 460 ng/ml, respectively. For Rac1 glucosylation, the EC50s of TcdB and TcdBF were 13 ng/ml and 220 ng/ml, respectively. Thus, TcdB was 1 order of magnitude more potent regarding substrate glucosylation and cytopathic effect than TcdBF.
Most recently, we and others showed that glucosylated RhoA and Cdc42 were more efficiently degraded by the proteasome than their nonmodified versions (12, 17). We exploited this fact to provide evidence on the cellular glucosylation of RhoA and Cdc42. Glucosylated RhoA was degraded in RBL cells exposed to increasing concentrations of TcdB, whereas the level of nonglucosylated RhoA from TcdBF-treated cells was unchanged (Fig. 1C). The cellular level of Cdc42 decreased in TcdB-treated cells, indicating its glucosylation. In contrast, a faint decrease of Cdc42 was observed at high concentrations of TcdBF, indicating (partial) glucosylation of Cdc42 (Fig. 1D). In conclusion, cellular RhoA, Rac1, and Cdc42 were glucosylated in TcdB-treated RBL cells; in TcdBF-treated RBL cells, Rac1 and Cdc42 (to a minor extent) were glucosylated, whereas RhoA remained unmodified.
Inactivation of Rho proteins is accompanied by up-regulation of the immediate early gene product RhoB (17, 18). This up-regulation was also observed in TcdB-treated RBL cells, as the level of RhoB increased in a concentration-dependent manner (Fig. 1E). In contrast, up-regulation of RhoB by TcdBF was less pronounced (Fig. 1E). RhoB is less efficiently glucosylated than RhoA; therefore, RhoB is active in toxin-treated cells and may be involved in the regulation of apoptosis (18).
Analysis of the cytotoxic effect induced by toxin B in RBL cells.
Inactivation of RhoA by Rho-inactivating toxins has been suggested to cause the cytotoxic effect in several cell lines (19, 31, 33). If inactivation of Rho was essential for the cytotoxic effect, one must expect TcdBF not to induce it. To check this possibility, activation of caspase-3 was analyzed in toxin-treated RBL cells. The level of active caspase-3 increased in a TcdB concentration-dependent manner, whereas TcdBF hardly induced caspase-3 activation (Fig. 2A), supporting the above idea that inactivation of RhoABC is crucial for the cytotoxic effect.
To analyze the cytotoxic effect at the single-cell level, phosphatidylserine exposure was recorded by annexin V staining. Data were presented as annexin V-positive cells per total cells. RBL cells were exposed to increasing concentrations of TcdB and TcdBF for 24 h. Both toxins induced the cytopathic effect, with TcdB being about 2 orders of magnitude more potent than TcdBF (Fig. 2B and C). TcdB induced an increase in annexin V-positive cells to a maximum of 40% (Fig. 2B), whereas the level of annexin V-positive cells increased only marginally after treatment with TcdBF (Fig. 2C). All cells, however, were sensitive to the cytotoxic effect induced by staurosporine, a potent broad-spectrum inhibitor of serine/threonine kinases (Fig. 2D). No increase in annexin V-positive cells was observed after treatment with latrunculin B, indicating that actin reorganization per se did not induce the cytotoxic effect (Fig. 2D). Although the complete population of RBL cells was sensitive to the cytopathic effect of TcdB and TcdBF, only 40% of the cells were sensitive to the cytotoxic effect of TcdB (Fig. 2B). Almost no cytotoxic effect was observed in TcdBF-treated cells (Fig. 2C).
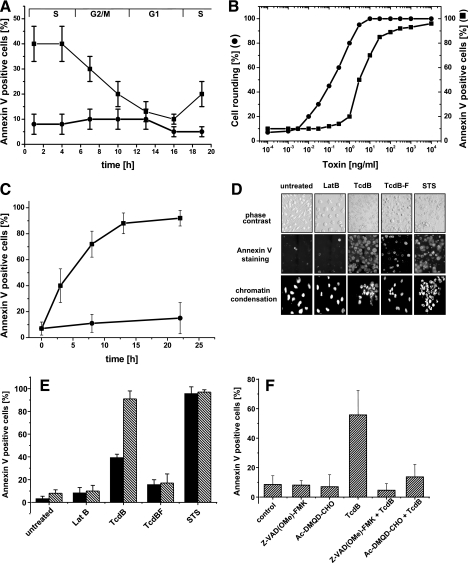
The onset of the execution phase of apoptosis is markedly asynchronous across a population of cells; therefore, we synchronized cells using the thymidine double-block technique (20, 32). This technique allowed the synchronization of more than 60% of the RBL cells in one phase of the cell cycle. The greatest clusterings of the cells were observed at 1 h (S population), 7 h (G2/M population), and 16 h (G1 population) after removal of the second block, as determined by DNA flow cytometry (Fig. 3A). To analyze the sensitivity of each population to the cytotoxic effects of TcdB and TcdBF, the toxins were applied to the cells at the indicated times (Fig. 3A). The cytotoxic effect was then analyzed after 3 h. RBL cells in S phase turned out to be most sensitive to the cytotoxic effect of TcdB (Fig. 3A). The sensitivity then decreased; after 19 h, the sensitivity increased again, as cells reentered S phase (Fig. 3A). We estimated that one cycle of RBL cells took 18 h; this matches data reported previously (17 h) (21). A marginal increase of the cytotoxic effect was observed in TcdBF-treated cells; the low sensitivity of TcdBF-treated cells to the cytotoxic effect was unchanged throughout the cell cycle (Fig. 3A). The latter observation excludes the possibility that TcdBF-treated cells were sensitive to a possible cytotoxic effect caused by inactivation of Ras proteins in another phase of the cell cycle.
FIG. 3.
Cytotoxic effect of TcdB in synchronized RBL cells. (A) Sensitivity of synchronized RBL cells to the cytotoxic effects of TcdB and TcdBF. After release from the thymidine double block, synchronized RBL cells were challenged with either TcdB (0.1 μg/ml) (▪) or TcdBF (1 μg/ml) (•) for phosphatidylserine exposure at the indicated times. Phosphatidylserine exposure was visualized by annexin V staining after 3 h of toxin treatment; the cytotoxic effect was quantified as annexin V-positive cells per total cells. (B) Cytotoxic effect of TcdB in synchronized RBL cells (concentration dependence). Synchronized RBL cells were exposed to TcdB for 24 h. The cytopathic effect was determined as percent rounded cells. Phosphatidylserine exposure was visualized by annexin V staining (▪); the cytotoxic effect was quantified as annexin V-positive per total cells. (C) Cytotoxic effect of TcdB in synchronized RBL cells (time dependence). Synchronized RBL cells were exposed to TcdB (0.1 μg/ml) for the periods indicated. Phosphatidylserine exposure was visualized by annexin V staining; the cytotoxic effect was quantified as annexin V-positive cells per total cells. Results are means of three independent experiments ± standard deviations. (D) Cytotoxic effect in synchronized RBL cells. Synchronized RBL cells were exposed to latrunculin B, staurosporine, TcdB, or TcdBF or left untreated for 24 h. Phosphatidylserine exposure was visualized by annexin V staining; chromatin condensation was analyzed by in situ DAPI staining of permeabilized cells. (E) Comparison of the cytotoxic effect in synchronized and nonsynchronized RBL cells. Synchronized (striped bars) or nonsynchronized (filled bars) RBL cells were exposed to latrunculin B (2.5 μM), staurosporine (0.2 μM), TcdB (0.1 μg/ml), or TcdBF (1 μg/ml) or left untreated for 24 h. Phosphatidylserine exposure was visualized by annexin V staining; the cytotoxic effect was quantified as percent annexin V-positive cells. Results are means of three independent experiments plus standard deviations. (F) Inhibition of the cytotoxic effect of TcdB by caspase inhibitors. Synchronized RBL cells were incubated with caspase-3 inhibitor (20 μM) or pancaspase inhibitor (20 μM) or left untreated for 1 h. TcdB (0.1 μg/ml) was then added, and incubation was continued for 6 h. Phosphatidylserine exposure was visualized by annexin V staining; the cytotoxic effect was quantified as percent annexin V-positive cells. Results are means of three independent experiments plus standard deviations.
The cytopathic effect and the cytotoxic effect were further analyzed in synchronized RBL cells treated with increasing concentrations of TcdB (Fig. 3B). The complete population of synchronized RBL cells was homogenously sensitive to both the cytopathic effect and the cytotoxic effect of TcdB (Fig. 3B). The glucosylation of RhoA, Rac1, and Cdc42 was also analyzed in synchronized cells (data not shown); the kinetics of glucosylation observed in synchronized cells was comparable to that observed in nonsynchronized cells (Fig. 1B to D).
The cytotoxic effect of TcdB was further analyzed in a time-dependent manner. In synchronized RBL cells, the half-maximal cytotoxic effect was reached after 5 to 6 h of TcdB treatment (Fig. 3C). In contrast, only a marginal cytotoxic effect was observed in TcdBF-treated RBL cells (Fig. 3C). In addition, synchronized cells were sensitive to the cytotoxic effect induced by staurosporine (Fig. 3D and E); actin reorganization induced by TcdBF or latrunculin B was accompanied by a marginal or no cytotoxic effect, respectively (Fig. 3D and E). The cytotoxic effect was also analyzed by the detection of fragmented nuclei. Most nuclei of synchronized RBL cells exposed to either TcdB or staurosporine were fragmented, whereas the nuclei of cells exposed to either TcdBF or latrunculin B remained intact (Fig. 3D). In conclusion, synchronized RBL cells were sensitive to the TcdB-induced cytotoxic effect, whereas TcdBF induced a marginal cytotoxic effect.
As shown in Fig. 2A, TcdB induced activation of caspase-3 in nonsynchronized RBL cells. The strong activation of caspase-3 (>15-fold in TcdB-treated compared to untreated cells) was also observed in synchronized RBL cells (data not shown). To confirm that caspases executed a cytotoxic effect, phosphatidylserine exposure was analyzed in RBL cells pretreated with either Z-VAD(OMe)-FMK, a broad-spectrum caspase inhibitor, or Ac-DMQD-CHO, an inhibitor of caspase-3. Both inhibitors blocked the cytotoxic effect; thus, caspase-3 essentially executed the cytotoxic effect of TcdB (Fig. 3F).
Viability of RBL cells is reduced after exposure to TcdB.
To further support the notion that TcdBF does not induce the cytotoxic effect, the viability of toxin-treated, synchronized RBL cells was assessed by WST-1 assay. Treatment of cells with either TcdBF or latrunculin B for 24 h induced the cytopathic effect but did not alter the viability of synchronized RBL cells (Fig. 4). In contrast, the viability of cells exposed to TcdB was reduced (Fig. 4). No viability was observed in staurosporine-treated cells (Fig. 4). These findings confirmed that TcdB was cytotoxic, while TcdBF was not.
DISCUSSION
The cellular effects of the glucosylating toxins are consequences of the inactivation of Rho and Ras proteins (25). Their most obvious effect is reorganization of the actin cytoskeleton (cytopathic effect) (9). Furthermore, the ability to induce apoptotic cell death (cytotoxic effect) has been reported for several glucosylating toxins (1, 5, 6, 13, 26, 37, 38).
Cultured cell lines are highly sensitive to the cytopathic effect but less sensitive to the cytotoxic effect (5, 13, 37). In this study, RBL cells were sensitized to the cytotoxic effect by synchronization using the thymidine double-block technique (20). The cytopathic effect was independent of cell synchronization, as the EC50s of the cytopathic effect in synchronized and nonsynchronized cells were comparable (Fig. 2B and 3B).
The cytotoxic effect of TcdB was analyzed in synchronized RBL cells in terms of phosphatidylserine exposure, fragmented nuclei, and activation of caspase-3. To our knowledge, this is the first report showing that a complete population of cells was affected by the cytotoxic effect. TcdB further induced the cytotoxic effect in synchronized murine fibroblasts and in macrophage-like J774A.1 cells (data not shown). Application of two caspase inhibitors completely blocked the cytotoxic effect of TcdB, confirming that it depended on the activity of caspase-3, which is in line with previous reports (19, 38); we did not observe a caspase-independent cytotoxic effect in synchronized RBL cells, as reported for TcdB-treated (nonsynchronized) HeLa cells (38).
A weak activation of caspase-3 and a slight increase in annexin V-positive cells were observed in TcdBF-treated RBL cells (this study), as well as in synchronized murine fibroblasts and macrophage-like J774A.1 cells (data not shown). The idea that TcdB (and not TcdBF) exhibits the cytotoxic effect is supported by a report showing that TcdB (and not TcdBF) causes DNA fragmentation in (nonsynchronized) endothelial cells (19). The viability of TcdBF-treated cells was comparable to that of latrunculin B-treated and control cells; thus, the cytopathic effect per se did not implicate the cytotoxic effect, a notion supported by numerous reports (13, 19, 37). Inactivation of RhoA, Rac1, and Cdc42 by TcdB efficiently induced both the cytopathic effect and cytotoxic effect in synchronized RBL cells; in contrast, inactivation of Rac1, Cdc42 (to a minor extent), and R-Ras by TcdBF resulted in the cytopathic effect and a marginal cytotoxic effect. Thus, Rac1 seems not to be crucial for either cell viability or the cytotoxic effect. Rac1 has been suggested to be the main regulator of the cytopathic effect (3). This suggestion is supported by our findings, as the cytopathic effect correlated with the glucosylation of Rac1 for either toxin (Fig. 1A and B).
We observed that S-phase cells were highly sensitive to the cytotoxic effect of TcdB, whereas cells in the other phases of the cell cycle were (partially) protected from the cytotoxic effect. In nonsynchronized RBL cells, 30 to 40% of total cells are present in S phase, and this subpopulation is seemingly sensitive to the cytotoxic effect of TcdB (Fig. 3D). The high sensitivity of S-phase cells to the cytotoxic effect of TcdB suggests that active Rho signaling is absolutely required for cellular survival in S phase; in other words, the cells rapidly undergo apoptosis in the absence of active Rho signaling. It is well documented for several cell lines, including murine EL4 lymphoma cells, that inactivation of RhoA but not of Rac1 or Cdc42 is required for the initiation of apoptosis (2, 19, 33, 34). Accordingly, inactivation of Rac1 by lethal toxin from Clostridium sordellii (TcsL) is not responsible for the cytotoxic effect in the myeloid cell line HL-60 (37). The latter observation, however, may not be true for neuronal cells, as inactivation of Rac1 by TcsL is shown to induce the cytotoxic effect (27, 29).
None of the studies cited was performed with synchronized cells; the ratio of apoptotic to total cells never exceeded 40%. Furthermore, the question of which phase of the cell cycle ectopically expressed RhoABC-inactivating exoenzyme C3 or N19RhoA exhibits the cytotoxic effect in remains to be answered (2, 33, 34). A target protein of TcdB possibly responsible for the sensitivity to the cytotoxic effect in S phase is RhoB. RhoB is a main regulator of apoptosis (14, 30); its expression pattern depends on the cell cycle and correlates with the sensitivity of the cells to the cytotoxic effect (Fig. 3A): RhoB is first detected at the G1/S phase transition; its level is maximal during the S phase and declines at the S/G2-M transition (46). Furthermore, rhoB has been suggested to be negatively regulated by RhoA as well as by RhoB itself (feedback inhibition) (15, 22, 23). Inactivation of cellular RhoA or RhoB by Rho-glucosylating toxins leads to desuppression of rhoB, resulting in a strong up-regulation of RhoB (17, 18); in this line, we observed a strong up-regulation of RhoB in TcdB-treated RBL cells in S phase (data not shown) as well as in nonsynchronized cells (Fig. 1E). Up-regulation of RhoB turned out to be less pronounced in TcdBF-treated cells (Fig. 1E); up-regulation of RhoB in TcdBF-treated cells is likely based on the inactivation of R-Ras, as Ras proteins have also been suggested to suppress rhoB (22, 23). Up-regulated RhoB is only partially glucosylated by TcdA and TcdB. In sum, an increased level of active RhoB is observed in TcdA- and TcdB-treated cells (compared to nontreated cells), which has led to the notion that the toxins activate RhoB (17, 18). The first evidence for a role of RhoB in the cytotoxic effect derives from the observation that inactivation of RhoB by cell-permeative C3 exoenzyme suppresses the cytotoxic effect of TcdB in S-phase fibroblasts; unfortunately, RBL cells were insensitive to the cell-permeative C3, forestalling this approach (unpublished observation). The application of rhoB small interfering RNA, however, will further elucidate the role of RhoB in the cytotoxic effect.
We identified TcdBF as the first isoform of the glucosylating toxins exhibiting the complete cytopathic effect but only a marginal cytotoxic effect. The protein substrate specificity of a toxin most likely determines whether it exhibits solely the cytopathic effect or additionally the cytotoxic effect: The inactivation of Rac1 by both TcdB and TcdBF is likely to result in the cytopathic effect; inactivation of RhoA by TcdB is accompanied by up-regulation of RhoB, which is most likely involved in the regulation of cytotoxic effect.
Variant strains of Clostridium difficile producing TcdB isoforms such as TcdBF or TcdB8864 (toxinotype A− B+) exhibit reduced pathogenicity in humans and in animal models compared to strains producing both TcdA and TcdB (toxinotype A+ B+) (4, 11). Our findings suggest that this reduced pathogenicity is based not solely on the absence of TcdA in the variant strains but also on the presence of TcdB isoforms (such as TcdBF) lacking the capability of inducing the cytotoxic effect.
Acknowledgments
We thank Ilona Klose for excellent technical assistance.
This work was supported by Deutsche Forschungsgemeinschaft priority program 1150 (project GE 1247/1-2) and SFB621 (project B5).
Editor: D. L. Burns
Footnotes
Published ahead of print on 4 December 2006.
REFERENCES
- 1.Agarwal, B., B. Halmos, A. S. Feoktistov, P. Protiva, W. G. Ramey, M. Chen, C. Pothoulakis, J. T. LaMont, and P. R. Holt. 2002. Mechanism of lovastatin-induced apoptosis in intestinal epithelial cells. Carcinogenesis 23:521-528. [DOI] [PubMed] [Google Scholar]
- 2.Bobak, D., J. Moorman, A. Guanzon, L. Gilmer, and C. Hahn. 1997. Inactivation of the small GTPase Rho disrupts cellular attachment and induces adhesion-dependent and adhesion-independent apoptosis. Oncogene 15:2179-2189. [DOI] [PubMed] [Google Scholar]
- 3.Boquet, P., and E. Lemichez. 2003. Bacterial virulence factors targeting Rho GTPases: parasitism or symbiosis? Trends Cell Biol. 13:238-246. [DOI] [PubMed] [Google Scholar]
- 4.Borriello, S. P., B. W. Wren, S. Hyde, S. V. Seddon, P. Sibbons, M. M. Krishna, S. Tabaqchali, S. Manek, and A. B. Price. 1992. Molecular, immunological, and biological characterization of a toxin A-negative, toxin B-positive strain of Clostridium difficile. Infect. Immun. 60:4192-4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brito, G. A., J. Fujji, B. A. Carneiro-Filho, A. A. Lima, T. Obrig, and R. L. Guerrant. 2002. Mechanism of Clostridium difficile toxin A-induced apoptosis in T84 cells. J. Infect. Dis. 186:1438-1447. [DOI] [PubMed] [Google Scholar]
- 6.Carneiro, B. A., J. Fujii, G. A. Brito, C. Alcantara, R. B. Oria, A. A. Lima, T. Obrig, and R. L. Guerrant. 2006. Caspase and Bid involvement in Clostridium difficile toxin A-induced apoptosis and modulation of toxin A effects by glutamine and alanyl-glutamine in vivo and in vitro. Infect. Immun. 74:81-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaves-Olarte, E., E. Freer, A. Parra, C. Guzmán-Verri, E. Moreno, and M. Thelestam. 2003. R-Ras glucosylation and transient RhoA activation determine the cytopathic effect produced by toxin B variants from toxin A-negative strains of Clostridium difficile. J. Biol. Chem. 278:7956-7963. [DOI] [PubMed] [Google Scholar]
- 8.Chaves-Olarte, E., P. Löw, E. Freer, T. Norlin, M. Weidmann, C. Von Eichel-Streiber, and M. Thelestam. 1999. A novel cytotoxin from Clostridium difficile serogroup F is a functional hybrid between two other large clostridial cytotoxins. J. Biol. Chem. 274:11046-11052. [DOI] [PubMed] [Google Scholar]
- 9.Chaves-Olarte, E., M. Weidmann, C. Von Eichel-Streiber, and M. Thelestam. 1997. Toxins A and B from Clostridium difficile differ with respect to enzymatic potencies, cellular substrate specificities, and surface binding to cultured cells. J. Clin. Investig. 100:1734-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coleman, M. L., C. J. Marshall, and M. F. Olson. 2004. RAS and RHO GTPases in G1-phase cell-cycle regulation. Nat. Rev. Mol. Cell. Biol. 5:355-366. [DOI] [PubMed] [Google Scholar]
- 11.Depitre, C., M. Delmee, V. Avesani, R. L'Haridon, A. Roels, M. Popoff, and G. Corthier. 1993. Serogroup F strains of Clostridium difficile produce toxin B but not toxin A. J. Med. Microbiol. 38:434-441. [DOI] [PubMed] [Google Scholar]
- 12.Dillon, S. T., E. J. Rubin, M. Yakubovich, C. Pothoulakis, J. T. LaMont, L. A. Feig, and R. J. Gilbert. 1995. Involvement of Ras-related Rho proteins in the mechanisms of action of Clostridium difficile toxin A and toxin B. Infect. Immun. 63:1421-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiorentini, C., A. Fabbri, L. Falzano, A. Fattorossi, P. Matarrese, R. Rivabene, and G. Donelli. 1998. Clostridium difficile toxin B induces apoptosis in intestinal cultured cells. Infect. Immun. 66:2660-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fritz, G., and B. Kaina. 2000. Ras-related GTPase RhoB forces alkylation-induced apoptotic cell death. Biochem. Biophys. Res. Commun. 268:784-789. [DOI] [PubMed] [Google Scholar]
- 15.Fritz, G., and B. Kaina. 1997. rhoB encoding a UV-inducible ras-related small GTP-binding protein is regulated by GTPases of the Rho family and independent of JNK, ERK, and p38 MAP kinase. J. Biol. Chem. 272:30637-30644. [DOI] [PubMed] [Google Scholar]
- 16.Genth, H., K. Aktories, and I. Just. 1999. Monoglucosylation of RhoA at threonine-37 blocks cytosol-membrane cycling. J. Biol. Chem. 274:29050-29056. [DOI] [PubMed] [Google Scholar]
- 17.Genth, H., J. Huelsenbeck, B. Hartmann, F. Hofmann, I. Just, and R. Gerhard. 2006. Cellular stability of Rho-GTPases glucosylated by Clostridium difficile toxin B. FEBS Lett. 580:3565-3569. [DOI] [PubMed] [Google Scholar]
- 18.Gerhard, R., H. Tatge, H. Genth, T. Thum, J. Borlak, G. Fritz, and I. Just. 2005. Clostridium difficile toxin A induces expression of the stress-induced early gene product RhoB. J. Biol. Chem. 280:1499-1505. [DOI] [PubMed] [Google Scholar]
- 19.Hippenstiel, S., B. Schmeck, P. D. N′Guessan, J. Seybold, M. Krüll, K. Preissner, C. Von Eichel-Streiber, and N. Suttorp. 2002. Rho protein inactivation induced apoptosis of cultured human endothelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 283:L830-L838. [DOI] [PubMed] [Google Scholar]
- 20.Hiroi, N., H. Maruta, and S. Tanuma. 1999. Fas-mediated apoptosis in Jurkat cells is suppressed in the pre-G2/M phase. Apoptosis 4:255-261. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda, K., B. Pant, A. Mishiro, K. Ozawa, T. Masujima, and M. Sugiyama. 2000. A convenient method for the evaluation of anti-tumor agents affecting the cell cycle. J. Biosci. Bioeng. 90:574-576. [PubMed] [Google Scholar]
- 22.Jiang, K., F. L. Delarue, and S. M. Sebti. 2004. EGFR, ErbB2 and Ras but not Src suppress RhoB expression while ectopic expression of RhoB antagonizes oncogene-mediated transformation. Oncogene 23:1136-1145. [DOI] [PubMed] [Google Scholar]
- 23.Jiang, K., J. Sun, J. Cheng, J.-Y. Djeu, S. Wei, and S. Sebti. 2004. Akt mediates Ras downregulation of RhoB, a suppressor of transformation, invasion, and metastasis. Mol. Cell. Biol. 24:5565-5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Just, I., and R. Gerhard. 2004. Large clostridial cytotoxins. Rev. Physiol. Biochem. Pharmacol. 152:23-47. [DOI] [PubMed] [Google Scholar]
- 25.Just, I., F. Hofmann, H. Genth, and R. Gerhard. 2001. Bacterial protein toxins inhibiting low-molecular-mass GTP-binding proteins. Int. J. Med. Microbiol. 291:243-250. [DOI] [PubMed] [Google Scholar]
- 26.Kim, H., E. Kokkotou, X. Na, S. H. Rhee, M. P. Moyer, C. Pothoulakis, and J. T. LaMont. 2005. Clostridium difficile toxin A-induced colonocyte apoptosis involves p53-dependent p21(WAF1/CIP1) induction via p38 mitogen-activated protein kinase. Gastroenterology 129:1875-1888. [DOI] [PubMed] [Google Scholar]
- 27.Le, S. S., F. A. Loucks, H. Udo, S. Richardson-Burns, R. A. Phelps, R. J. Bouchard, H. Barth, K. Aktories, K. L. Tyler, E. R. Kandel, K. A. Heidenreich, and D. A. Linseman. 2005. Inhibition of Rac GTPase triggers a c-Jun and Bim-dependent mitochondrial apoptotic cascade in cerebellar granule neurons. J. Neurochem. 94:1025-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lees, P., J. Giraudel, M. F. Landoni, and P. L. Toutain. 2004. PK-PD integration and PK-PD modelling of nonsteroidal anti-inflammatory drugs: principles and applications in veterinary pharmacology. J. Vet. Pharmacol. Ther. 27:491-502. [DOI] [PubMed] [Google Scholar]
- 29.Linseman, D. A., T. Laessig, M. K. Meintzer, M. McClure, H. Barth, K. Aktories, and K. A. Heidenreich. 2001. An essential role for Rac/Cdc42 GTPases in cerebellar granule neuron survival. J. Biol. Chem. 276:39123-39131. [DOI] [PubMed] [Google Scholar]
- 30.Liu, A., G. J. Cerniglia, E. J. Bernhard, and G. C. Prendergast. 2001. RhoB is required to mediate apoptosis in neoplastically transformed cells after DNA damage. Proc. Natl. Acad. Sci. USA 98:6192-6197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahida, Y. R., A. Galvin, S. Makh, S. Hyde, L. Sanfilippo, S. P. Borriello, and H. F. Sewell. 1998. Effect of Clostridium difficile toxin A on human colonic lamina propria cells: early loss of macrophages followed by T-cell apoptosis. Infect. Immun. 66:5462-5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mills, J. C., N. L. Stone, J. Erhardt, and R. N. Pittman. 1998. Apoptotic membrane blebbing is regulated by myosin light chain phosphorylation. J. Cell Biol. 140:627-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moorman, J. P., D. A. Bobak, and C. S. Hahn. 1996. Inactivation of the small GTP binding protein Rho induces multinucleate cell formation and apoptosis in murine T lymphoma EL4. J. Immunol. 156:4146-4153. [PubMed] [Google Scholar]
- 34.Moorman, J. P., D. Luu, J. Wickham, D. A. Bobak, and C. S. Hahn. 1999. A balance of signaling by Rho family small GTPases RhoA, Rac1 and Cdc42 coordinates cytoskeletal morphology but not cell survival. Oncogene 18:47-57. [DOI] [PubMed] [Google Scholar]
- 35.Otsu, K., K. Sato, Y. Ikeda, H. Imai, Y. Nakagawa, Y. Ohba, and J. Fujii. 2005. An abortive apoptotic pathway induced by singlet oxygen is due to the suppression of caspase activation. Biochem. J. 389:197-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pederzoli, M., C. Kantari, V. Gausson, S. Moriceau, and V. Witko-Sarsat. 2005. Proteinase-3 induces procaspase-3 activation in the absence of apoptosis: potential role of this compartmentalized activation of membrane-associated procaspase-3 in neutrophils. J. Immunol. 174:6381-6390. [DOI] [PubMed] [Google Scholar]
- 37.Petit, P., J. Bréard, V. Montalescot, N. B. El Hadj, T. Levade, M. Popoff, and B. Geny. 2003. Lethal toxin from Clostridium sordellii induces apoptotic cell death by disruption of mitochondrial homeostasis in HL-60 cells. Cell. Microbiol. 5:761-771. [DOI] [PubMed] [Google Scholar]
- 38.Qa'Dan, M., M. Ramsey, J. Daniel, L. M. Spyres, B. Safiejko-Mroczka, W. Ortiz-Leduc, and J. D. Ballard. 2002. Clostridium difficile toxin B activates dual caspase-dependent and caspase-independent apoptosis in intoxicated cells. Cell. Microbiol. 4:425-434. [DOI] [PubMed] [Google Scholar]
- 39.Raftopoulou, M., and A. Hall. 2004. Cell migration: Rho GTPases lead the way. Dev. Biol. 265:23-32. [DOI] [PubMed] [Google Scholar]
- 40.Rupnik, M., N. Kato, M. Grabnar, and H. Kato. 2003. New types of toxin A-negative, toxin B-positive strains among Clostridium difficile isolates from Asia. J. Clin. Microbiol. 41:1118-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sehr, P., G. Joseph, H. Genth, I. Just, E. Pick, and K. Aktories. 1998. Glucosylation and ADP-ribosylation of Rho proteins—effects on nucleotide binding, GTPase activity, and effector-coupling. Biochemistry 37:5296-5304. [DOI] [PubMed] [Google Scholar]
- 42.Tatton, L., G. M. Morley, R. Chopra, and A. Khwaja. 2003. The Src-selective kinase inhibitor PP1 also inhibits Kit and Bcr-Abl tyrosine kinases. J. Biol. Chem. 278:4847-4853. [DOI] [PubMed] [Google Scholar]
- 43.Vetter, I. R., F. Hofmann, S. Wohlgemuth, C. Herrmann, and I. Just. 2000. Structural consequences of mono-glucosylation of Ha-Ras by Clostridium sordellii lethal toxin. J. Mol. Biol. 301:1091-1095. [DOI] [PubMed] [Google Scholar]
- 44.Voth, D. E., and J. Ballard. 2005. Clostridium difficile Toxins: mechanism of action and role in disease. Clin. Microbiol. Rev. 18:247-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wennerberg, K., and C. J. Der. 2004. Rho-family GTPases: it's not only Rac and Rho (and I like it). J. Cell Sci. 117:1301-1312. [DOI] [PubMed] [Google Scholar]
- 46.Zalcman, G., V. Closson, G. Linarès-Cruz, F. Lerebours, N. Honoré, A. Tavitian, and B. Olofsson. 1995. Regulation of Ras-related RhoB protein expression during the cell cycle. Oncogene 10:1935-1945. [PubMed] [Google Scholar]