Abstract
Current experimental vaccines against serogroup B Neisseria meningitidis are based on meningococcal outer membrane (OM) proteins present in outer membrane vesicles (OMV) in which toxic lipopolysaccharide is depleted by detergent extraction. Knowledge of the composition of OM and OMV is essential for developing new meningococcal vaccines based on defined antigens. In the current study, sodium dodecyl sulfate-polyacrylamide gel electrophoresis and nanocapillary liquid chromatography-tandem mass spectrometry were used to investigate the proteomes of OM and OMV from meningococcal strain MC58 and OM from a lipopolysaccharide-deficient mutant. The analysis of OM revealed a composition that was much more complex than the composition that has been reported previously; a total of 236 proteins were identified, only 6.4% of which were predicted to be located in the outer membrane. The most abundant proteins included not only the well-established major OM proteins (PorA, PorB, Opc, Rmp, and Opa) but also other proteins, such as pilus-associated protein Q (PilQ) and a putative macrophage infectivity protein. All of these proteins were also present in OMV obtained by extraction of the OM with deoxycholate. There were markedly increased levels of some additional proteins in OM from the lipopolysaccharide-deficient mutant, including enzymes that contribute to the tricarboxylic acid cycle. In all the preparations, the proteins not predicted to have an OM location were predominantly periplasmic or cytoplasmic or had an unknown location, and relatively few cytoplasmic membrane proteins were detected. However, several proteins that have previously been identified as potential vaccine candidates were not detected in either OM preparations or in OMV. These results have important implications for the development and use of vaccines based on outer membrane proteins.
Neisseria meningitidis (meningococci) causes life-threatening meningitis and septicemia, principally in infants and adolescents. The recent development of vaccines comprising capsular polysaccharide from serogroup C meningococci conjugated to carrier proteins has led to a substantial decline in serogroup C infections in countries where the vaccine has been adopted (2). Similar strategies to produce conjugate vaccines containing serogroup A, W135, and Y polysaccharides are also likely to succeed. However, the capsular polysaccharide from serogroup B strains, which have been the predominant strains in most temperate countries (2), exhibits structural similarities to human neural cell adhesion molecules and is nonimmunogenic in humans (13). Therefore, for development of an effective vaccine against serogroup B meningococci workers have focused on the proteins of the outer membrane (OM).
Isolation of outer membrane fractions from gram-negative bacteria is usually based on isolation of a whole-membrane fraction, followed by separation into discrete outer membrane and cytoplasmic membrane components by density gradient centrifugation (30). Such procedures have proved to be ineffective for isolation of outer membranes from Neisseria species (22). Therefore, for alternative isolation methods workers have utilized the observation that unlike the outer membranes of enteric bacteria, the Neisseria outer membrane can be detached as “blebs” by procedures that apply mild shear forces without disrupting the rest of the cell (14, 32). Such procedures include the “shake and bake” method, where the bacteria are shaken at 45°C in lithium acetate in the presence of glass beads (18, 50). Analysis of such preparations by one-dimensional sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) revealed that they contained lipopolysaccharide (LPS) and a restricted number of proteins that are believed to reside in the outer membrane (14). The current experimental and newly licensed vaccines against serogroup B meningococcal infection are based on outer membrane vesicles (OMV) obtained by extraction with deoxycholate detergent to remove LPS (14), which is responsible for induction of the acute inflammatory response and tissue destruction characteristic of meningococcal infection (7). More recently, mutants of meningococci lacking LPS have been created by insertional inactivation of the lpxA gene, which encodes the enzyme required for the first step in the biosynthesis of the lipid A moiety of LPS (47). Outer membranes isolated from such mutants represent an alternative source of material for vaccine use.
Studies of such vaccines have shown that the immune responses to different components are different in different individuals and that only a proportion of the antibodies induced are protective (27, 36). However, a full understanding of the basis of the immune response is hampered by the lack of detailed knowledge concerning the composition of meningococcal OM blebs and the OMV derived from them. Studies to determine the protein compositions of these structures have been limited to analysis by one-dimensional SDS-PAGE and analysis by probing immunoblots with a panel of antibodies specific for known components of the outer membrane of N. meningitidis (1, 46) The availability of meningococcal genome sequences, including that of strain MC58 (48), together with improvements in proteomic techniques now enables workers to use a more direct approach to determine the protein compositions of both OM and OMV preparations.
Proteome analyses are conventionally carried out using two-dimensional (2D) gel electrophoresis, followed by mass spectrometric (MS) protein identification. Although 2D gels are able to resolve complex protein mixtures, low-abundance proteins and components with extreme physicochemical properties are difficult to detect. In particular, a major limitation of the technique is the poor solubility of membrane proteins, which consequently are underrepresented on 2D gels (37). SDS-PAGE and nanocapillary liquid chromatography-tandem mass spectrometry (GeLC-MS/MS) constitute an alternative approach which involves one-dimensional (1D) electrophoretic separation of proteins based on their sizes, followed by liquid chromatography and tandem MS. Because this approach effectively solubilizes and fractionates membrane proteins while sample complexity is reduced, it can significantly increase the number of peptides that are identified and assigned to proteins (41). An additional advantage of GeLC-MS/MS is that it provides an indication of relative abundance based on the number of peptide fragments identified per protein (5, 34, 35).
In this study we took advantage of the recent sequencing of the complete genome of N. meningitidis strain MC58 (48) and used GeLC-MS/MS to analyze the protein compositions of normal and deoxycholate-extracted OM derived from strain MC58, as well as OM derived from an isogenic mutant deficient in LPS. The results obtained indicate that such preparations are unexpectedly complex, which has important implications for vaccine studies.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
N. meningitidis MC58 (serogroup B; serotype 15; serosubtype P1.7,16b) has been described previously (25) and is the serogroup B strain whose genome sequence is available (48). An LPS-deficient mutant of strain MC58 was produced by insertional inactivation of the lpxA gene as described by Steeghs et al. (47), and the absence of LPS was confirmed by SDS-PAGE (40) and silver staining (21) and by the Limulus assay (Sigma). Bacteria were grown on proteose peptone agar plates in the presence of 5% (vol/vol) CO2 at 37°C for 16 to 18 h.
Preparation of outer membranes and vesicles.
Meningococcal cells were harvested from confluent growth of bacteria on 60 14.5-cm plates directly into 0.2 M lithium acetate (pH 5.8) and extracted at 45°C in the presence of glass beads, and the outer membranes were recovered by differential centrifugation as described previously (49). OMV with LPS depleted were prepared from the same batches of OM preparations (5 mg ml−1) by extraction with 1% (wt/vol) sodium deoxycholate in 1 mM Tris-HCl buffer (pH 8.5) containing 10 mM EDTA (8). The protein concentrations of the preparations were determined with a bicinchoninic acid protein assay kit (Pierce Biotechnology, Rockford, IL) used according to the manufacturer's instructions.
SDS-PAGE.
SDS-PAGE of outer membrane preparations was carried out on linear 10 to 25% acrylamide gradients, and the gels were stained with Coomassie blue as previously described (19). 2D electrophoresis was carried out with 100-μg OM preparations as described by Bernardini et al. (3), and proteins were visualized by silver staining with a ProteoSilver Plus staining kit (Sigma) used according to the manufacturer's instructions.
SDS-PAGE and GeLC-MS/MS.
One-dimensional SDS-polyacrylamide gel electrophoresis coupled with GeLC-MS/MS was used as described previously (41) to identify proteins associated with OM and OMV preparations. Preparations containing 100 μg of protein were solubilized in 0.1% (wt/vol) octyl glucoside and fractionated (30 μg/track) on a NuPAGE 4 to 12% SDS-polyacrylamide gradient gel (Invitrogen). After visualization with colloidal Coomassie blue, each gel lane (length, 7 cm) was excised, cut into 29 pieces that were the same size, and subjected to in situ trypsin digestion using the method of Shevchenko et al. (43). The resulting peptides were separated by nano reversed-phase liquid chromatography, using a Waters C18 column (3 μm; 100 Å; 150 mm by 75 μm [inside diameter]), and electrosprayed into a quadrupole time-of-flight tandem mass spectrometer.
Mass spectrometry and data processing.
All data were acquired using a Q-tof Global Ultima (Waters Ltd.) fitted with a nanoLockSpray source to achieve a mass accuracy that was better than 10 ppm. A survey scan was acquired from m/z 375 to 1800 with the switching criteria for MS to MS/MS including (i) ion intensity, (ii) charge state, and (iii) an exclusion list. The exclusion list was generated using the real-time database searching algorithm in ProteinLynx Global Server 2.05 (Waters Ltd.). The collision energy used to perform MS/MS was varied according to the mass and charge state of the eluting peptide.
All MS/MS spectra were automatically processed and searched against a FASTA-formatted list of protein sequences predicted from the MC58 genome and subsequently against the NCBI nonredundant database (June 2005 version), using ProteinLynx Global Server 2.05. Proteins were assigned only if, for each peptide ion, three or more experimentally derived y ions could be matched to the predicted spectra. When only one peptide was used in the identification of a specific protein, manual assignment of the spectra was performed. When peptides matched more than one database entry due to redundant protein sequence submissions, assignments to the duplicated sequence were removed.
In silico characterization of proteins.
The predicted physicochemical parameters and subcellular locations of the proteins identified in this study were determined using protein sequences extracted from the MC58 genomic database located at http://www.tigr.org/. Theoretical isoelectric pH (pI) and molecular weight values were calculated using the ProteinLynx Global Server package (version 2.05).
Computational prediction of subcellular locations from the amino acid sequence information was performed using PSORTb, version 2.0 (15) (located at http://psort.org/).
RESULTS
Meningococcal outer membrane fractions.
The outer membrane preparations were analyzed by 1D SDS-PAGE, and there were a limited number of major bands (Fig. 1) that were very similar to bands described previously by us and by other investigators (51). The OM fraction yielded three major bands, which on the basis of Mr corresponded to PorA, PorB, and Opa. The OMV fraction produced the same profile of major bands, but the number of minor bands was reduced. In contrast, the intensities of a number of minor bands from the OM from the LPS-negative mutant were increased; these bands included bands at apparent Mr of approximately 100,000 and 39,000. 2D gel electrophoresis of the OM preparation from wild-type strain MC58 resulted in approximately 330 spots on silver-stained gels (not shown).
FIG. 1.
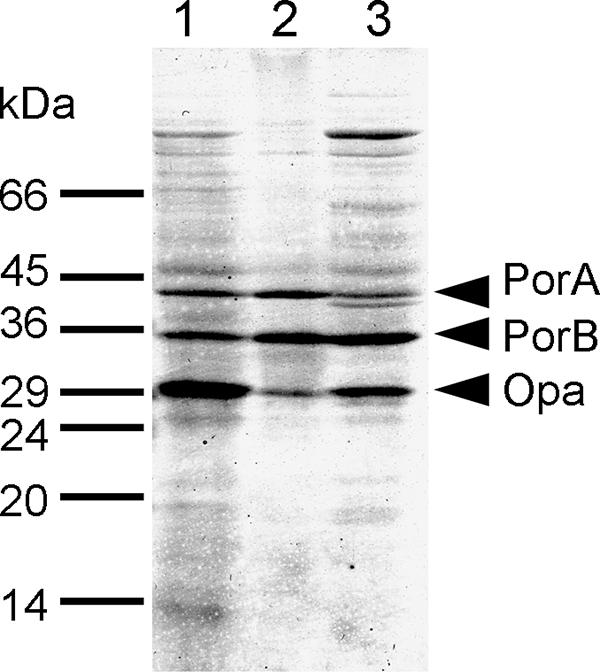
1D SDS-PAGE of outer membrane preparations from meningococcal strain MC58. Lane 1, OM from the wild type; lane 2, OMV preparation; lane 3, OM from the LPS-deficient mutant.
GeLC-MS/MS analysis of OM fractions. (i) Wild-type OM.
To obtain a detailed catalogue of constituent proteins, OM preparations were subjected to GeLC-MS/MS. Three replicate tryptic digests were analyzed for each preparation, and the results were matched with the tryptic peptides predicted from a protein sequence database derived from MC58 meningococcal genome data. A total of 666 peptide assignments were made, resulting in identification of 236 nonredundant proteins. Approximately 50% of the proteins were identified on the basis of two or more peptides, while the remainder were matched with single peptides using stringent identification criteria (see Materials and Methods). The identified proteins were correlated on the basis of their predicted locations within the bacterial cell using the PSORTb algorithm, which predicts the subcellular locations of proteins in gram-negative bacteria according to the presence or absence of leader peptides, homologies to known proteins, transmembrane domains, and outer membrane anchoring motifs (15). A total of 15 proteins were predicted by the PSORTb analysis to be located in the outer membrane (Table 1) ; these proteins represented only 6.4% of the total number of proteins detected in this fraction.
TABLE 1.
Predicted meningococcal OM proteins identified in this studya
| Category or protein nameb | NCBI locus tag (NMB no.) | No. of peptides in:
|
Molecular mass (kDa)c | pIc | Predicted function (COGS)d | ||
|---|---|---|---|---|---|---|---|
| OM | OMV | LPS− OM | |||||
| PorA outer membrane protein | 1429 | 16 | 26 | 10 | 42.1 | 9.3 | Cell wall/membrane biogenesis |
| PorB outer membrane proteine | 2039 | 14 | 20 | 13 | 35.7 | 7.8 | Cell wall/membrane biogenesis |
| Opc proteinf | 1053 | 9 | 16 | 7 | 30.0 | 10.0 | Not in COGS |
| PilQ protein | 1812 | 8 | 10 | 4 | 82.4 | 9.8 | Intracellular trafficking and secretion |
| Macrophage infectivity potentiator | 1567 | 5 | 2 | 9 | 28.9 | 5.8 | Posttranslational modification, protein turnover, and chaperones |
| Rmp proteine | 0382 | 4 | 7 | 2 | 26.2 | 7.6 | Cell wall/membrane biogenesis |
| Cell binding factor, putative | 0345 | 4 | 3 | 3 | 31.4 | 9.5 | Posttranslational modification, protein turnover, and chaperones |
| Outer membrane protein Omp85 | 0182 | 3 | 10 | 5 | 88.4 | 8.9 | Cell wall/membrane biogenesis |
| Multidrug efflux pump channel protein | 1714 | 2 | 7 | 1 | 50.4 | 8.6 | Cell wall/membrane biogenesis |
| Outer membrane protein P1, putative | 0088 | 2 | 3 | 1 | 50.5 | 9.6 | Lipid transport and metabolism |
| Adhesion and penetration protein | 1985 | 2 | 2 | 1 | 159.9 | 9.4 | Cell wall/membrane biogenesis |
| Organic solvent tolerance protein, putative | 0280 | 1 | 3 | 0 | 84.2 | 8.9 | Cell wall/membrane biogenesis |
| Outer membrane protein NspAg | 0663 | 1 | 2 | 0 | 18.4 | 9.8 | Cell wall/membrane biogenesis |
| VacJ-related protein | 1961 | 1 | 0 | 0 | 29.5 | 4.8 | Cell wall/membrane biogenesis |
| TbpBh | 0460 | 1 | 0 | 0 | 77.4 | 6.0 | Not in COGS |
| TbpAh | 0461 | 0 | 3 | 1 | 102.0 | 9.6 | Inorganic ion transport and metabolism |
| Maltose phosphorylase | 0390 | 0 | 2 | 2 | 85.4 | 5.5 | Carbohydrate transport and metabolism |
| TonB-dependent receptor | 1497 | 0 | 2 | 0 | 104.2 | 9.6 | Inorganic ion transport and metabolism |
| Conserved hypothetical protein | 2134 | 0 | 2 | 0 | 69.8 | 8.6 | Cell wall/membrane biogenesis |
| Phospholipase A1 putative | 0464 | 0 | 1 | 0 | 42.9 | 8.9 | Cell wall/membrane biogenesis |
| Conserved hypothetical protein | 1333 | 0 | 1 | 0 | 65.7 | 10.3 | Cell cycle control, mitosis, and meiosis |
| Lactoferrin binding protein A | 1540 | 0 | 1 | 0 | 105.6 | 9.6 | Inorganic ion transport and metabolism |
| Serine type peptidase | 1998 | 0 | 1 | 0 | 157.6 | 9.2 | Cell wall/membrane biogenesis |
| Conserved hypothetical protein | 2050 | 0 | 1 | 0 | 66.6 | 5.5 | Unknown |
| Cardiolipin synthetase family protein | 1434 | 0 | 1 | 0 | 57.3 | 9.3 | Lipid transport and metabolism |
| Phosphoribosylformylglycinamidine synthase | 1996 | 0 | 0 | 1 | 143.8 | 5.4 | Nucleotide transport and metabolism |
Proteins predicted by the PSORTb algorithm (located at http://psort.org/) to have an outer membrane location which were detected in the OM preparations.
The protein name is the name in the annotation of the MC58 genome located at http://www.tigr.org/ unless indicated otherwise.
The theoretical molecular mass and pI values were calculated using the ProteinLynx Global Server Package (version 2.05).
Predicted functional categories were derived from the Clusters of Orthologous Groups of proteins (COGS) database (located at http://www.ncbi.nlm.nih.gov/sutils/). Similar results were obtained in an analysis of a second independent preparation of each fraction.
The name in the annotation of the MC58 genome located at http://www.tigr.org/ differs from the previously published name used in the text. See reference 20.
The name in the annotation of the MC58 genome located at http://www.tigr.org/ differs from the previously published name used in the text. See reference 29.
See reference 24.
The name in the annotation of the MC58 genome located at http://www.tigr.org/ differs from the previously published name used in the text. See reference 42.
The predominant outer membrane proteins detected were those that have been well established as the major components of the outer membrane, namely, the porins PorA and PorB and the Opc, PilQ, and Rmp proteins (20, 51). The major exception was the Opa protein, which was clearly detected by 1D SDS-PAGE. The proteins not predicted to have an outer membrane location were predominantly periplasmic or cytoplasmic or had an unknown location, and relatively few cytoplasmic membrane proteins were detected (Table 2).
TABLE 2.
Predominant proteins detected in the outer membrane fractions that are not predicted to have an outer membrane locationa
| Category or protein nameb | NCBI locus tag (NMB no.) | No. of peptides in:
|
Molecular mass (kDa)c | pIc | PSORTb locationd | Predicted function (COGS)e | ||
|---|---|---|---|---|---|---|---|---|
| OM | OMV | LPS− OM | ||||||
| Pyruvate dehydrogenase E1 component | 1341 | 24 | 11 | 24 | 99.5 | 5.8 | U | Energy production and conversion |
| Chaperonin (60 kDa) | 1972 | 22 | 15 | 24 | 57.4 | 4.9 | C | Posttranslational modification, protein turnover, and chaperones |
| Glutamate dehydrogenase, NADP specific | 1710 | 16 | 9 | 12 | 48.5 | 6.2 | C | Amino acid transport and metabolism |
| Glyceraldehyde-3-phosphate dehydrogenase | 2159 | 14 | 6 | 5 | 35.8 | 5.5 | C | Carbohydrate transport and metabolism |
| ATP synthase F1 alpha subunit | 1936 | 12 | 7 | 3 | 55.3 | 5.5 | U | Energy production and conversion |
| Pyruvate dehydrogenase E2 component dihydrolipoamide acetyltransferase | 1342 | 11 | 3 | 4 | 55.2 | 5.4 | C | Energy production and conversion |
| DNA-directed RNA polymerase beta subunit | 0132 | 9 | 8 | 32 | 155.8 | 5.4 | C | Transcription |
| Enolase | 1285 | 9 | 5 | 8 | 46.1 | 4.8 | C | Carbohydrate transport and metabolism |
| Bacterioferritin A | 1207 | 9 | 2 | 5 | 17.9 | 4.8 | C | Inorganic ion transport and metabolism |
| GNA1946f | 1946 | 9 | 1 | 7 | 31.2 | 5.2 | U | Inorganic ion transport and metabolism |
| ATP synthase F1 beta subunit | 1934 | 8 | 7 | 5 | 50.4 | 5.0 | C | Energy production and conversion |
| Polyribonucleotide nucleotidyltransferase | 0758 | 8 | 3 | 13 | 76.4 | 5.4 | C | Translation |
| IMP dehydrogenase | 1201 | 8 | 3 | 8 | 52.4 | 7.2 | C | Nucleotide transport and metabolism |
| Transketolase | 1457 | 7 | 3 | 12 | 71.6 | 5.6 | U | Carbohydrate transport and metabolism |
| ATP synthase F1 gamma subunit | 1935 | 7 | 1 | 3 | 32.5 | 9.3 | U | Energy production and conversion |
| Oligopeptidase A | 0214 | 6 | 13 | 8 | 76.0 | 5.2 | C | Amino acid transport and metabolism |
| Translation elongation factor Tu | 0139 | 6 | 10 | 7 | 42.9 | 5.1 | C | Translation |
| Translation elongation factor Tu | 0124 | 6 | 6 | 5 | 42.9 | 5.1 | C | Translation |
| Amino acid ABC transporter periplasmic amino acid binding protein | 1612 | 6 | 1 | 5 | 27.0 | 5.1 | P | Amino acid transport and metabolism |
| 5-Methyltetrahydropteroyltriglutamate homocysteine methyltransferase | 0944 | 5 | 9 | 6 | 85.0 | 5.4 | C | Amino acid transport and metabolism |
| Conserved hypothetical protein | 0109 | 5 | 7 | 0 | 50.1 | 9.3 | U | Unknown |
| DNA-directed RNA polymerase beta subunit | 0133 | 5 | 5 | 16 | 153.7 | 7.3 | C | Transcription |
| Alcohol dehydrogenase, propanol preferring | 0546 | 5 | 3 | 11 | 36.5 | 5.9 | C | General function prediction only |
| 50S ribosomal protein L1 | 0128 | 5 | 3 | 7 | 24.1 | 9.9 | U | Translation |
| 2-Oxoglutarate dehydrogenase E2 component dihydrolipoamide succinyltransferase | 0956 | 5 | 3 | 6 | 41.5 | 5.2 | CM | Energy production and conversion |
| Thiol disulfide interchange protein DsbA | 0294 | 5 | 1 | 7 | 25.4 | 5.4 | P | Posttranslational modification, protein turnover, and chaperones |
| ATP phosphoribosyltransferase | 1579 | 5 | 1 | 5 | 23.2 | 8.7 | C | Amino acid transport and metabolism |
| Adhesin complex protein, putative | 2095 | 5 | 1 | 5 | 13.3 | 9.8 | U | Not in COGS |
| Spermidine putrescine ABC transporter periplasmic spermidine putrescine binding protein | 0623 | 5 | 1 | 3 | 41.2 | 5.8 | P | Amino acid transport and metabolism |
| Tryptophan synthase beta subunit | 0699 | 5 | 1 | 2 | 43.2 | 6.6 | C | Amino acid transport and metabolism |
| Hypothetical protein | 1126 | 4 | 8 | 2 | 23.8 | 8.9 | U | Not in COGS |
| Iron III ABC transporter periplasmic binding protein | 0634 | 4 | 5 | 2 | 35.8 | 9.9 | P | Inorganic ion transport and metabolism |
| Carbamoyl phosphate synthase large subunit | 1855 | 4 | 4 | 10 | 117.3 | 5.2 | U | Amino acid transport and metabolism |
| Pilin PilE | 0018 | 4 | 2 | 2 | 18.1 | 9.4 | U | Cell motility |
| Spermidine putrescine ABC transporter periplasmic spermidine putrescine binding protein | 0462 | 4 | 2 | 1 | 50.5 | 7.5 | P | Amino acid transport and metabolism |
| 2-Oxoglutarate dehydrogenase E3 component lipoamide dehydrogenase | 0957 | 4 | 1 | 10 | 50.1 | 6.2 | C | Energy production and conversion |
| Thiol disulfide interchange protein DsbA | 0278 | 4 | 1 | 4 | 25.2 | 5.7 | U | Posttranslational modification, protein turnover, and chaperones |
| Conserved hypothetical protein | 1652 | 4 | 1 | 3 | 46.4 | 5.0 | U | Unknown |
| Bacterioferritin B | 1206 | 4 | 0 | 4 | 18.0 | 4.7 | C | Inorganic ion transport and metabolism |
| Conserved hypothetical protein | 1590 | 4 | 0 | 4 | 11.5 | 7.5 | U | Unknown |
| H.8 outer membrane protein | 1533 | 4 | 0 | 2 | 18.5 | 4.7 | P | Energy production and conversion |
| Elongation factor G | 0138 | 3 | 8 | 7 | 77.2 | 5.1 | C | Translation |
| DnaK protein | 0554 | 3 | 8 | 4 | 68.7 | 4.9 | U | Posttranslational modification, protein turnover, and chaperones |
| Valyl tRNA synthetase | 0174 | 3 | 4 | 7 | 106.6 | 5.3 | C | Translation |
| Acetate kinase | 1518 | 3 | 4 | 4 | 42.4 | 6.1 | C | Energy production and conversion |
| Argininosuccinate lyase | 0637 | 3 | 3 | 5 | 51.2 | 5.3 | C | Amino acid transport and metabolism |
| Formate tetrahydrofolate ligase | 1839 | 3 | 3 | 5 | 59.0 | 6.1 | C | Nucleotide transport and metabolism |
| Aldehyde dehydrogenase A | 1968 | 3 | 2 | 12 | 52.2 | 5.3 | C | Energy production and conversion |
| Hypothetical protein | 0928 | 3 | 2 | 2 | 43.8 | 9.3 | P | Cell wall/membrane biogenesis |
| Thiol disulfide interchange protein DsbC | 0550 | 3 | 2 | 2 | 28.6 | 8.7 | P | Posttranslational modification, protein turnover, and chaperones |
| Delta-aminolevulinic acid dehydratase | 0801 | 3 | 1 | 5 | 36.8 | 5.3 | C | Coenzyme transport and metabolism |
| Ketol acid reductoisomerase | 1574 | 3 | 1 | 4 | 36.4 | 5.9 | U | Amino acid transport and metabolism |
| DNA-directed RNA polymerase alpha subunit | 0168 | 3 | 1 | 3 | 36.1 | 5.0 | C | Transcription |
| Glutamate ammonia ligase | 0359 | 3 | 1 | 3 | 52.1 | 5.3 | C | Amino acid transport and metabolism |
| Malate oxidoreductase, NAD | 0671 | 3 | 1 | 3 | 45.4 | 5.1 | U | Energy production and conversion |
| Thiol disulfide interchange protein DsbD | 1519 | 3 | 1 | 1 | 64.9 | 6.7 | CM | Posttranslational modification, protein turnover, and chaperones |
| Hypothetical protein | 1468 | 3 | 0 | 5 | 10.8 | 4.6 | U | Not in COGS |
| RNA polymerase sigma factor RpoD | 1538 | 3 | 0 | 3 | 73.8 | 4.9 | C | Transcription |
| Host factor I | 0748 | 3 | 0 | 3 | 10.8 | 8.8 | U | General function prediction only |
| Transsulfuration enzyme family protein | 1609 | 3 | 0 | 2 | 41.9 | 6.7 | C | Amino acid transport and metabolism |
| d-Lactate dehydrogenase | 0997 | 3 | 0 | 0 | 63.4 | 6.7 | C | Energy production and conversion |
| ClpB protein | 1472 | 2 | 7 | 4 | 95.1 | 5.5 | C | Posttranslational modification, protein turnover, and chaperones |
| Ribonucleoside diphosphate reductase alpha subunit | 1291 | 2 | 4 | 3 | 85.0 | 6.2 | C | Nucleotide transport and metabolism |
| 50S ribosomal protein L4 | 0143 | 2 | 4 | 0 | 23.2 | 10.2 | U | Translation |
| 50S ribosomal protein L10 | 0130 | 2 | 3 | 0 | 17.6 | 8.6 | U | Translation |
| Succinyl-coenzyme A synthetase beta subunit | 0959 | 2 | 2 | 6 | 41.3 | 5.1 | C | Energy production and conversion |
| Isoleucyl tRNA synthetase | 1833 | 2 | 2 | 5 | 104.1 | 5.6 | C | Translation |
| Glucose-6-phosphate isomerase | 1388 | 2 | 2 | 4 | 62.0 | 6.4 | C | Carbohydrate transport and metabolism |
| Enoyl acyl carrier protein reductase | 0336 | 2 | 1 | 6 | 27.7 | 6.1 | C | Lipid transport and metabolism |
| Lipoprotein, putative | 0204 | 2 | 1 | 4 | 13.9 | 9.1 | U | Translation |
| 50S ribosomal protein L3 | 0142 | 2 | 1 | 3 | 22.7 | 10.4 | C | Translation |
| Threonyl tRNA synthetase | 0720 | 2 | 1 | 3 | 72.6 | 6.2 | C | Translation |
| Peptidyl prolyl cis,trans-isomerase | 0791 | 2 | 1 | 3 | 16.8 | 5.1 | C | Posttranslational modification, protein turnover, and chaperones |
| Acetolactate synthase III large subunit | 1577 | 2 | 1 | 3 | 62.8 | 6.2 | U | Amino acid transport and metabolism |
| Conserved hypothetical protein | 1796 | 2 | 1 | 3 | 20.9 | 8.9 | U | General function prediction only |
| Gamma-glutamyltranspeptidase | 1057 | 2 | 1 | 3 | 65.0 | 6.3 | P | Amino acid transport and metabolism |
| Single-strand binding protein | 1460 | 2 | 0 | 4 | 19.4 | 6.0 | U | Replication, recombination, and repair |
| Aspartate carbamoyltransferase regulatory subunit | 0107 | 2 | 0 | 3 | 16.9 | 9.3 | C | Nucleotide transport and metabolism |
| DNA binding protein HU beta | 1230 | 2 | 0 | 3 | 9.3 | 9.8 | U | Replication, recombination, and repair |
| Amino acid ABC transporter periplasmic amino acid binding protein | 0787 | 2 | 0 | 3 | 28.8 | 6.2 | P | Amino acid transport and metabolism |
| Argininosuccinate synthase | 2129 | 1 | 8 | 3 | 49.6 | 5.2 | U | Amino acid transport and metabolism |
| Hemolysin, putative | 2091 | 1 | 3 | 5 | 21.7 | 10.0 | U | General function prediction only |
| Leucyl tRNA synthetase | 1897 | 1 | 3 | 2 | 98.0 | 5.2 | U | Not in COGS |
| 50S ribosomal protein L19 | 0589 | 1 | 3 | 1 | 13.8 | 10.7 | C | Translation |
| 30S ribosomal protein S1 | 1301 | 1 | 3 | 0 | 61.1 | 5.0 | C | Translation |
| Carboxy terminal peptidase | 1332 | 1 | 3 | 0 | 53.2 | 9.4 | U | Cell wall/membrane biogenesis |
| Citrate synthase | 0954 | 1 | 1 | 8 | 48.1 | 6.7 | C | Energy production and conversion |
| Pyruvate dehydrogenase E3 component lipoamide dehydrogenase | 1344 | 1 | 1 | 8 | 61.8 | 5.1 | C | Energy production and conversion |
| Conserved hypothetical protein | 1073 | 1 | 1 | 5 | 42.0 | 4.6 | U | Amino acid transport and metabolism |
| Proline dehydrogenase | 0401 | 1 | 1 | 4 | 129.9 | 6.4 | C | Amino acid transport and metabolism |
| Phosphoenolpyruvate synthase | 0618 | 1 | 1 | 3 | 87.1 | 5.1 | C | Carbohydrate transport and metabolism |
| Rare lipoprotein B, putative | 0707 | 1 | 1 | 3 | 17.6 | 6.5 | U | Cell wall/membrane biogenesis |
| Conserved hypothetical protein | 0317 | 1 | 0 | 5 | 18.1 | 5.9 | C | General function prediction only |
| Alcohol dehydrogenase, zinc containing | 0604 | 1 | 0 | 5 | 37.9 | 5.7 | U | Amino acid transport and metabolism |
| 2,3,4,5-Tetrahydropyridine-2-carboxylate N-succinyltransferase | 0335 | 1 | 0 | 4 | 29.4 | 5.5 | C | Amino acid transport and metabolism |
| Hypothetical protein | 0478 | 1 | 0 | 4 | 23.6 | 5.0 | U | Not in COGS |
| Phosphoribosylaminoimidazole carboxylase catalytic subunit | 1439 | 1 | 0 | 4 | 17.1 | 5.5 | U | Nucleotide transport and metabolism |
| Polypeptide deformylase | 0110 | 1 | 0 | 3 | 19.1 | 5.8 | C | Translation |
| Cysteine synthase | 0763 | 1 | 0 | 3 | 32.8 | 6.4 | U | Amino acid transport and metabolism |
| Phosphate acetyltransferase Pta, putative | 0631 | 0 | 8 | 2 | 52.2 | 4.8 | U | General function prediction only |
| Trigger factor | 1313 | 0 | 4 | 1 | 48.3 | 4.7 | U | Posttranslational modification, protein turnover, and chaperones |
| Soluble lytic murein transglycosylase, putative | 1949 | 0 | 4 | 0 | 67.7 | 9.5 | P | Cell wall/membrane biogenesis |
| Peroxiredoxin two-family protein glutaredoxin | 0946 | 0 | 3 | 1 | 26.9 | 4.8 | U | Posttranslational modification, protein turnover, and chaperones |
| Membrane fusion protein | 1716 | 0 | 3 | 0 | 42.8 | 9.2 | CM | Cell wall/membrane biogenesis |
| Glyceraldehyde-3-phosphate dehydrogenase | 0207 | 0 | 1 | 3 | 38.9 | 8.6 | C | Carbohydrate transport and metabolism |
| Chorismate synthase | 1680 | 0 | 1 | 3 | 39.4 | 6.5 | C | Amino acid transport and metabolism |
| Adenylosuccinate lyase | 0284 | 0 | 1 | 3 | 50.4 | 5.9 | U | Nucleotide transport and metabolism |
| 2-Oxoglutarate dehydrogenase E1 component | 0955 | 0 | 0 | 8 | 105.0 | 6.6 | C | Energy production and conversion |
| Glutamate dehydrogenase, NAD specific | 1476 | 0 | 0 | 4 | 46.2 | 5.3 | C | Amino acid transport and metabolism |
| DNA topoisomerase I | 0118 | 0 | 0 | 3 | 87.0 | 8.2 | C | Replication, recombination, and repair |
| AspAg | 1029 | 0 | 0 | 3 | 50.7 | 5.4 | C | Amino acid transport and metabolism |
| Phosphoenolpyruvate carboxylase | 2061 | 0 | 0 | 3 | 101.1 | 6.8 | C | Energy production and conversion |
| Conserved hypothetical protein | 1653 | 0 | 0 | 3 | 10.4 | 5.0 | U | Signal transduction mechanisms |
| Cytochrome c5 | 1677 | 0 | 0 | 3 | 28.7 | 6.2 | U | Energy production and conversion |
Proteins which were detected on the basis of three or more peptide matches and which were predicted by PSORTb to have locations other than the outer membrane.
The protein name is the name in the annotation of the MC58 genome located at http://www.tigr.org/ unless indicated otherwise.
The theoretical molecular mass and pI values were calculated using the ProteinLynx Global Server Package (version 2.05).
Subcellular location predicted by PSORTb. C, cytoplasmic; P, periplasmic; CM, cytoplasmic membrane; U, unattributed. Similar results were obtained in an analysis of a second independent preparation of each fraction. Proteins detected with only one or two peptide matches are included in the full complement of proteins detected, which is available in the supplemental material.
Predicted functional categories were derived from the Clusters of Orthologous Groups of proteins (COGS) database (located at http://www.ncbi.nlm.nih.gov/sutils/). Similar results were obtained in an analysis of a second independent preparation of each fraction.
The name in the annotation of the MC58 genome located at http://www.tigr.org/ differs from the published name used in the text. See reference 33.
The name in the annotation of the MC58 genome located at http://www.tigr.org/ differs from the published name used in the text. See reference 52.
(ii) Comparison of OMV and OM from the LPS mutant with wild-type OM.
GeLC-MS/MS analysis of the OMV fraction with LPS depleted produced a total of 602 peptide assignments, resulting in identification of 223 nonredundant proteins. Although this number was similar to the number of proteins detected in the wild-type OM preparation, there were some differences in the proteins detected. In general, a number of the predicted cytoplasmic proteins that were present at low levels in the OM preparation were not detected in the OMV preparation, while eight additional OM proteins, which were not found in the OM preparation, were detected at low levels in OMV (Tables 1 and 2).
In the case of the OM from the LPS-deficient mutant, 848 spectra were collected, resulting in identification of 292 unique proteins, which was 56 more proteins than the number of proteins in the wild-type OM preparation. The majority of the additional proteins detected were cytoplasmic proteins (Table 2). Only one protein predicted by PSORTb to be located in the OM was unique to this preparation (phosphoribosylformylglycinamidine synthase), but it was detected on the basis of only one peptide (Table 1). Based on Mr and the increase in the number of peptides detected, the two proteins whose intensities were significantly increased on 2D gels were tentatively identified as the 2-oxoglutarate dehydrogenase E1 component (NMB0955; molecular mass, 105 kDa) and zinc-containing alcohol dehydrogenase (NMB0604; molecular mass, 38 kDa) (Table 2).
Detection of Opa proteins.
The OM preparation used in these studies was prepared from the parent strain of MC58 originally described (25) and clearly contained a single Opa protein as a major component (Fig. 1). This protein was not detected by searching the MS/MS spectra obtained against a list of protein sequences predicted from the MC58 genome database. However, no Opa proteins are annotated in the translation of the MC58 genome (39). In order to identify Opa and any other proteins corresponding to nonannotated sequences, a BLAST search of the peptides detected was carried out against a translation of all six frames of the genome sequence (45). Only two additional protein hits obtained with high levels of significance (E values) and more than two peptides per protein were identified on the basis of similarity with entries in the Swiss-Prot database. One protein exhibited strong homology with the Opa proteins from serogroup C meningococcal strain FAM18 and the related gonococcal Opa proteins from strain FA1090, confirming the presence of Opa in the OM preparation. The other protein exhibited homology with a yeast hypothetical protein with an unknown function. Similar results were obtained with the OMV and OM preparations from the LPS-negative mutant.
DISCUSSION
In a previous study using 2D electrophoresis with whole-cell extracts of a serogroup A meningococcal strain and a pI range of 3 to 10 workers reported detection of 273 unique proteins, including 94 proteins that were previously only hypothetical proteins, corresponding to approximately 13% of the 2,121 open reading frames predicted from the genome sequence of the strain (3). In a similar study using strain MC58 but a restricted pI range (pI 4 to 7), the workers found 238 unique proteins (26). In the current study, in which preliminary experiments were performed with strain MC58 using 2D electrophoresis of OM with a pI range of 3 to 10, 330 spots were detected, which is considerably more than the number that might have been expected if the preparation had contained only proteins located in the outer membrane. However, a significant number of meningococcal proteins have been reported to appear as multiple electrophoretic species on 2D gels due to the presence of isoforms whose pIs and molecular weights vary (3).
The GeLC-MS/MS method used in the current study increases the number of proteins that can be identified in a relatively unbiased manner (41, 44). An additional advantage is that the number of peptides detected per protein permits workers to estimate the relative abundance (5, 34), and it has been suggested that the relationship may be linear (35). In this study 26 (49%) of the 53 genomic outer membrane proteins predicted by PSORTb were detected. The most abundant of these included proteins with important biological functions, including the PorA and PorB porins, the Opc protein associated with invasion of epithelial and endothelial cells (56), the Rmp protein, which may protect against complement-mediated bactericidal attack (28), and the PilQ protein, which is essential for pilus assembly (11). An additional outer membrane protein was found among the major components. NMB1567 is annotated on the MC58 genome as a “macrophage infectivity potentiator” (Mip) due to its 65% homology with an equivalent protein from Legionella londiniensis (48). Furthermore, the Legionella Mip protein, which exhibits peptidylprolyl cis,trans-isomerase activity, has been reported to play an important role in virulence, perhaps through receptor recognition or inhibition of host defenses (16). No direct role for a meningococcal Mip has been determined, but its presence in the outer membrane is clearly potentially important.
The proteins not predicted to have an outer membrane location were predominantly periplasmic or cytoplasmic or had an unknown location, and relatively few cytoplasmic membrane proteins were detected, suggesting that the outer membrane fraction is composed of OM proteins together with periplasmic and cytoplasmic components but contains little cytoplasmic membrane. In general, the cytosolic components that were detected corresponded to proteins involved in housekeeping functions. This is not surprising as such proteins tend to be most abundant in this subcellular compartment.
OMV preparations used as experimental vaccines are produced by deoxycholate treatment to deplete the toxic LPS in the OM. They can be produced either by initial isolation of OM followed by deoxycholate extraction or, alternatively, by treatment of whole cells with the detergent (14). In the current study, the OMV preparation used was produced by extraction of the same wild-type OM preparation, and the catalogue of proteins identified was similar but not identical; comparatively more OM proteins and fewer cytoplasmic proteins were detected in the OMV. It is likely that the deoxycholate extraction of OM resulted in solubilization and removal of some cytoplasmic proteins, hence increasing the levels of other proteins above a threshold detection level. In contrast, all of the proteins identified as major outer membrane proteins remained present at high levels in OMV, and the number of peptides detected generally reflected enrichment compared with OM. In the current study it was not possible to determine whether the “non-outer membrane” proteins, particularly periplasmic proteins, occurred in a natural association with the OM and OMV preparations or represented “contamination” of the preparation. However similar results were obtained when we analyzed a second independent OM preparation by GeLC-MS/MS. While it likely that different methods of isolation might influence the protein profile of OM and OMV preparations, in a recent 2D electrophoresis analysis of OMV prepared by deoxycholate extraction of whole bacteria, workers reported that only 34 of the 138 proteins that could be identified on 2D gels were assigned by PSORT to outer membrane locations (12). Similarly, 186 proteins were identified in an OMV vaccine preparation from Neisseria lactamica, and most of them would be expected to have non-outer membrane locations (53). While it is possible that direct extraction of bacteria results in inclusion of some additional proteins, the current study revealed that both OM blebs and the OMV derived from them inherently contain a large number of such proteins.
Although present in all three membrane preparations, the Opa protein was noticeably absent from the proteins detected by GeLC-MS/MS. In other recent studies with meningococcal OMV the workers also did not find Opa (53, 54). Opa proteins are major components of the meningococcal outer membrane and play an important role in colonization and invasion of host epithelial cells (55), but they are not vaccine candidates due to their phase variation, which may enable meningococci to evade at least some of the consequences of the normal host immune response (49). A single meningococcus strain may express up to four Opa proteins, each of which is subject to independent phase variation. Phase variation results from reversible mutations in the number of five nucleotide repeat sequences that occur within the open reading frame, which result in on/off switches in expression of the protein. It is therefore possible that in other studies workers have used OMV prepared from an Opa-negative variant. In the current study we used the original isolate of strain MC58 (25), and Opa was demonstrated to be a major component of the OM preparation but was not detected in the initial interrogation of the MC58 genome database. However, the published genome sequence was obtained from an Opa-negative variant of MC58, and each of the four Opa open reading frames present is out of frame for expression in the sequenced variant (39). By using an alternative strategy, a BLAST search of the peptides detected against a translation of all six frames of the genome sequence, we were able to confirm the presence of Opa both in OM preparations and in OMV. Interestingly, no other known meningococcal phase-variable proteins were detected by this method.
Immunization of human subjects with experimental OMV-based vaccines has been shown to induce antibodies that promote complement-mediated killing of meningococci, the accepted correlate of protection against invasive meningococcal disease (4, 6, 10, 31). Because of the heterologous nature of OMV preparations, antibodies were directed against different antigens, the relative responses varied for different individuals, and only a proportion of the antibodies were protective. The only protein antigens that have been identified so far as antigens that contribute to the bactericidal activity following immunization of humans with OMV are the PorA (27), PorB, and Opc proteins (36), although Opc is absent from many disease isolates (38) and the biological significance of anti-PorB antibodies remains uncertain (36). All three of these proteins were found to be major components of the OM and OMV in the current study. More recently, a number of alternative proteins have been proposed as vaccine candidates. These include the Omp85 protein (23), which was one of the most abundant proteins in the OMV preparation in the current study and was predicted to be located in the outer membrane by PSORTb. In contrast, several other putative vaccine candidates were not detected in the OMV, including AspA (52), GNA2132 NadA (9), GNA1870, and GNA2001 (33), while NspA (24) was detected. Most of these proteins were also not detected in the OM fraction from which the OMV were derived by detergent extraction. The single exception was GNA1870, which was detected at low levels in the OM fraction, and it is possible that deoxycholate extraction may have reduced the amount of the protein to below the level of detection. While the failure to detect a protein cannot be taken as absolute evidence of absence, the semiquantitative nature of GeLC-MS/MS suggests that proteins that are not detected are most likely to be present at low levels However, with the exception of NspA, the latter proteins were predicted by PSORTb to have locations other than the outer membrane. In recent studies with OMV obtained by direct deoxycholate extraction of the bacteria the workers also did not report the presence of these antigens (53, 54). It is possible that such proteins are not integral outer membrane proteins but subsequently associate with the outer surface of the bacteria, presenting potential targets for bactericidal antibodies, and hence remain potential vaccine targets. However, in a study using 2D electrophoresis the workers did not report finding these proteins in the whole-cell proteome of strain MC58 (26). It is also interesting that none of seven putative toxins (48) identified in the genome of strain MC58 were present in either OM or OMV fractions. Definitive knowledge of the OM and OMV proteomes should allow systematic, directed studies to determine which other proteins induce antibodies that promote complement-mediated killing of meningococci.
The potential use as a vaccine of an outer membrane fraction from an LPS-deficient meningococcal strain is dependent on retaining potentially protective proteins in an immunogenic form. However, the absence of LPS has additional effects on the properties of the outer membrane (46). Mutants without LPS contain phospholipids with shorter and saturated fatty acid acyl chains and have a reduced growth rate and increased susceptibility to hydrophobic antibiotics. The major difference in protein expression between another wild-type serogroup B meningococcal strain and an LPS mutant reported previously was decreased expression of LbpB and TbpB (46). These lipoproteins are expressed under iron-limited growth conditions and form parts of the lactoferrin and transferrin receptor, respectively. No attempt was made to limit the available iron source in the current study. Consequently, as expected, the LbpB and TbpB proteins were absent or present at low levels in OM prepared from both parent and mutant strains. However, for the most part the major proteins present in the OM from the parent strain were present at similar levels in the mutant, although one proposed vaccine candidate, NspA, was not detected in LPS-deficient OM. NspA has been suggested as a vaccine candidate based on its degree of conservation between strains and the observation that monoclonal antibodies directed against it are bactericidal (24), although in a recent study bactericidal antibodies were not detected in humans following immunization with purified NspA (17). Interestingly, the levels of several proteins were markedly increased in the OM preparation from the LPS-deficient mutant; these proteins included the enzymes citrate synthase, 2-oxaloglutarate dehydrogenase, and succinyl-coenzyme A synthetase. This observation suggests that there is increased flux through the tricarboxylic acid cycle in the LPS-negative mutant. However, further studies are necessary to determine the precise biochemical rationale for the up-regulation of this metabolic module.
GeLC-MS/MS has provided a means to obtain detailed information concerning the composition of meningococcal outer membrane preparations, and studies have revealed a composition that is much more complex than the composition that has been reported previously. In particular, data have demonstrated the presence of, and provided information on the relative levels of, minor components in an OMV preparation of the type that forms the basis of current experimental vaccines directed against serogroup B strains. In addition, data have provided important information concerning an alternative to OMV for putative vaccine preparations and concerning the most comprehensive analysis of the protein content of the outer envelope of an LPS-deficient meningococcal strain. Such information should be important in studies on the human immune response to such vaccines and for identification of antigens that may contribute to a protective immune response.
Supplementary Material
Acknowledgments
This work was principally supported by Hope (The Wessex Medical Trust) along with contributions from The Meningitis Research Foundation and The University of Southampton Strategic Development Fund.
We are grateful to Therese Nestor for excellent technical assistance and to P. van der Ley and L. Steeghs for providing plasmid pLAK33 used in the construction of the MC58 LPS-negative mutant.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 11 December 2006.
Supplemental material for this article may be found at http://iai.asm.org/.
REFERENCES
- 1.Andersen, S. R., G. Bjune, E. A. Hoiby, T. E. Michaelsen, A. Aase, U. Rye, and E. Jantzen. 1997. Outer membrane vesicle vaccines made from short-chain lipopolysaccharide mutants of serogroup B Neisseria meningitidis: effect of the carbohydrate chain length on the immune response. Vaccine 15:1225-1234. [DOI] [PubMed] [Google Scholar]
- 2.Balmer, P., R. Borrow, and E. Miller. 2002. Impact of meningococcal C conjugate vaccine in the UK. J. Med. Microbiol. 51:717-722. [DOI] [PubMed] [Google Scholar]
- 3.Bernardini, G., G. Renzone, M. Comanducci, R. Mini, S. Arena, C. D'Ambrosio, S. Bambini, L. Trabalzini, G. Grandi, P. Martelli, M. Achtman, A. Scaloni, G. Ratti, and A. Santucci. 2004. Proteome analysis of Neisseria meningitidis serogroup A. Proteomics 4:2893-2926. [DOI] [PubMed] [Google Scholar]
- 4.Bjune, G., E. A. Hoiby, J. K. Gronnesby, O. Arnesen, J. Holstfredriksen, A. Halstensen, E. Holten, A. K. Lindbak, H. Nokleby, E. Rosenqvist, L. K. Solberg, O. Closs, J. Eng, L. O. Froholm, A. Lystad, L. S. Bakketeig, and B. Hareide. 1991. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 338:1093-1096. [DOI] [PubMed] [Google Scholar]
- 5.Blondeau, F., B. Ritter, P. D. Allaire, S. Wasiak, M. Girard, N. K. Hussain, A. Angers, V. Legendre-Guillemin, L. Roy, D. Boismenu, R. E. Kearney, A. W. Bell, J. J. Bergeron, and P. S. McPherson. 2004. Tandem MS analysis of brain clathrin-coated vesicles reveals their critical involvement in synaptic vesicle recycling. Proc. Natl. Acad. Sci. USA 101:3833-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boslego, J., J. Garcia, C. Cruz, W. Zollinger, B. Brandt, S. Ruiz, M. Martinez, J. Arthur, P. Underwood, W. Silva, E. Moran, W. Hankins, J. Gilly, and J. Mays. 1995. Efficacy, safety, and immunogenicity of a meningococcal group B (15 P1.3) outer membrane protein vaccine in Iquique, Chile. Vaccine 13:821-829. [DOI] [PubMed] [Google Scholar]
- 7.Brandtzaeg, P., R. Ovstebo, and P. Kierulf. 1992. Compartmentalization of lipopolysaccharide production correlates with clinical presentation in meningococcal disease. J. Infect. Dis. 166:650-652. [DOI] [PubMed] [Google Scholar]
- 8.Christodoulides, M., J. L. Brooks, E. Rattue, and J. E. Heckels. 1998. Immunisation with recombinant class 1 outer membrane protein from Neisseria meningitidis: influence of liposomes and adjuvants on antibody avidity, recognition of native protein and the induction of a bactericidal immune response against meningococci. Microbiology 144:3027-3037. [DOI] [PubMed] [Google Scholar]
- 9.Comanducci, M., S. Bambini, D. A. Caugant, M. Mora, B. Brunelli, B. Capecchi, L. Ciucchi, R. Rappuoli, and M. Pizza. 2004. NadA diversity and carriage in Neisseria meningitidis. Infect. Immun. 72:4217-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Moraes, J. C., B. A. Perkins, M. C. C. Camargo, N. T. R. Hidalgo, H. A. Barbosa, C. T. Sacchi, I. M. L. Gral, V. L. Gattas, H. D. Vasconcelos, B. D. Plikaytis, J. D. Wenger, and C. V. Broome. 1992. Protective efficacy of a serogroup B meningococcal vaccine in Sao Paulo, Brazil. Lancet 340:1074-1078. [DOI] [PubMed] [Google Scholar]
- 11.Drake, S. L., and M. Koomey. 1995. The product of the pilQ gene is essential for the biogenesis of type IV pili in Neisseria gonorrhoeae. Mol. Microbiol. 18:975-986. [DOI] [PubMed] [Google Scholar]
- 12.Ferrari, G., I. Garaguso, J. Adu-Bobie, F. Doro, A. R. Taddei, B. Biolchi, B. Brunelli, M. M. Giuliani, M. Pizza, N. Norais, and G. Grandi. 2006. Outer membrane vesicles from group B Neisseria meningitidis gna33 mutant: proteomic and immunological comparison with detergent-derived outer membrane vesicles. Proteomics 6:1856-1866. [DOI] [PubMed] [Google Scholar]
- 13.Finne, J., M. Leinonen, and P. H. Makela. 1983. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet ii:355-357. [DOI] [PubMed] [Google Scholar]
- 14.Frasch, C. E., L. van Alphen, J. Holst, J. T. Poolman, and E. Rosenqvist. 2001. Outer membrane protein vesicle vaccines for meningococcal disease, p. 81-107. In A. J. Pollard and M. C. J. Maiden (ed.) Meningococcal vaccines. Humana Press, Totowa, NJ. [DOI] [PubMed]
- 15.Gardy, J. L., M. R. Laird, F. Chen, S. Rey, C. J. Walsh, M. Ester, and F. S. Brinkman. 2005. PSORTb v. 2.0: expanded prediction of bacterial protein subcellular localization and insights gained from comparative proteome analysis. Bioinformatics 21:617-623. [DOI] [PubMed] [Google Scholar]
- 16.Hacker, J., and G. Fischer. 1993. Immunophilins—structure-function relationship and possible role in microbial pathogenicity. Mol. Microbiol. 10:445-456. [DOI] [PubMed] [Google Scholar]
- 17.Halperin, S. A., J. M. Langley, B. Smith, P. Wunderli, L. Kaufman, A. Kimura, and D. Martin. 2007. Phase 1 first-in-human studies of the reactogenicity and immunogenicity of a recombinant NspA vaccine in healthy adults. Vaccine 25:450-457. [DOI] [PubMed] [Google Scholar]
- 18.Heckels, J. E. 1977. The surface properties of Neisseria gonorrhoeae: isolation of the major components of the outer membrane. J. Gen. Microbiol. 99:333-341. [DOI] [PubMed] [Google Scholar]
- 19.Heckels, J. E. 1981. Structural comparison of Neisseria gonorrhoeae outer membrane proteins. J. Bacteriol. 145:736-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hitchcock, P. J. 1989. Unified nomenclature for pathogenic Neisseria species. Clin. Microbiol. Rev. 2:S64-S65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hitchcock, P. J., and T. M. Brown. 1983. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J. Bacteriol. 154:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnston, K. H., K. K. Holmes, and E. C. Gotschlich. 1976. The serological classification of Neisseria gonorrhoeae. I. Isolation of the outer membrane complex responsible for serotype specificity. J. Exp. Med. 143:741-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Judd, R. C. 2004. Identification of surface epitopes of neisserial outer membrane protein 85, p. 166. In M. A. Apicella and H. S. Seifert (ed.), Proceedings of the 14th International Pathogenic Neisseria Conference, Milwaukee, WI.
- 24.Martin, D., N. Cadieux, J. Hamel, and B. R. Brodeur. 1997. Highly conserved Neisseria meningitidis surface protein confers protection against experimental infection. J. Exp. Med. 185:1173-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGuinness, B. T., I. N. Clarke, P. R. Lambden, A. K. Barlow, J. T. Poolman, D. M. Jones, and J. E. Heckels. 1991. Point mutation in meningococcal porA gene associated with increased endemic disease. Lancet 337:514-517. [DOI] [PubMed] [Google Scholar]
- 26.Mignogna, G., A. Giorgi, P. Stefanelli, A. Neri, G. Colotti, B. Maras, and M. E. Schinina. 2005. Inventory of the proteins in Neisseria meningitidis serogroup B strain MC58. J. Proteome Res. 1361:1370. [DOI] [PubMed] [Google Scholar]
- 27.Milagres, L. G., S. R. Ramos, C. T. Sacchi, C. E. A. Melles, V. S. D. Vieira, H. Sato, G. S. Brito, J. C. Moraes, and C. E. Frasch. 1994. Immune response of Brazilian children to a Neisseria meningitidis serogroup B outer membrane protein vaccine: comparison with efficacy. Infect. Immun. 62:4419-4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munkley, A., C. R. Tinsley, M. Virji, and J. E. Heckels. 1991. Blocking of bactericidal killing of Neisseria meningitidis by antibodies directed against class 4 outer membrane protein. Microb. Pathog. 11:447-452. [DOI] [PubMed] [Google Scholar]
- 29.Olyhoek, A. J. M., J. Sarkari, M. Bopp, G. Morelli, and M. Achtman. 1991. Cloning and expression in Escherichia coli of opC, the gene for an unusual class 5 outer membrane protein from Neisseria meningitidis (meningococci surface-antigen). Microb. Pathog. 11:249-257. [DOI] [PubMed] [Google Scholar]
- 30.Osborn, M. J., and R. Munson. 1974. Separation of the inner (cytoplasmic) and outer membranes of Gram-negative bacteria. Methods Enzymol. 31:642-653. [DOI] [PubMed] [Google Scholar]
- 31.Perkins, B. A., K. Jonsdottir, H. Briem, E. Griffiths, B. D. Plikaytis, E. A. Hoiby, E. Rosenqvist, J. Holst, H. Nokleby, F. Sotolongo, G. Sierra, H. C. Campa, G. M. Carlone, D. Williams, J. Dykes, D. Kapczynski, E. Tikhomirov, J. D. Wenger, and C. V. Broome. 1998. Immunogenicity of two efficacious outer membrane protein-based serogroup B meningococcal vaccines among young adults in Iceland. J. Infect. Dis. 177:683-691. [DOI] [PubMed] [Google Scholar]
- 32.Pettit, R. K., and R. C. Judd. 1992. Characterization of naturally elaborated blebs from serum-susceptible and serum-resistant strains of Neisseria gonorrhoeae. Mol. Microbiol. 6:723-728. [DOI] [PubMed] [Google Scholar]
- 33.Pizza, M., V. Scarlato, V. Masignani, M. M. Giuliani, B. Arico, M. Comanducci, G. T. Jennings, L. Baldi, E. Bartolini, B. Capecchi, C. L. Galeotti, E. Luzzi, R. Manetti, E. Marchetti, M. Mora, S. Nuti, G. Ratti, L. Santini, S. Savino, M. Scarselli, E. Storni, P. J. Zuo, M. Broeker, E. Hundt, B. Knapp, E. Blair, T. Mason, H. Tettelin, D. W. Hood, A. C. Jeffries, N. J. Saunders, D. M. Granoff, J. C. Venter, E. R. Moxon, G. Grandi, and R. Rappuoli. 2000. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 287:1816-1820. [DOI] [PubMed] [Google Scholar]
- 34.Qian, W. J., T. Liu, M. E. Monroe, E. F. Strittmatter, J. M. Jacobs, L. J. Kangas, K. Petritis, D. G. Camp II, and R. D. Smith. 2005. Probability-based evaluation of peptide and protein identifications from tandem mass spectrometry and SEQUEST analysis: the human proteome. J. Proteome Res. 4:53-62. [DOI] [PubMed] [Google Scholar]
- 35.Ritter, B., F. Blondeau, A. Y. Denisov, K. Gehring, and P. S. McPherson. 2004. Molecular mechanisms in clathrin-mediated membrane budding revealed through subcellular proteomics. Biochem. Soc. Trans. 32:769-773. [DOI] [PubMed] [Google Scholar]
- 36.Rosenqvist, E., E. A. Hoiby, E. Wedege, K. Bryn, J. Kolberg, A. Klem, E. Ronnild, G. Bjune, and H. Nokleby. 1995. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect. Immun. 63:4642-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santoni, V., M. Molloy, and T. Rabilloud. 2000. Membrane proteins and proteomics: un amour impossible? Electrophoresis 21:1054-1070. [DOI] [PubMed] [Google Scholar]
- 38.Sarkari, J., N. Pandit, E. R. Moxon, and M. Achtman. 1994. Variable expression of the Opc outer membrane protein in Neisseria meningitidis is caused by size variation of a promoter containing poly-cytidine. Mol. Microbiol. 13:207-217. [DOI] [PubMed] [Google Scholar]
- 39.Saunders, N. J., A. C. Jeffries, J. F. Peden, D. W. Hood, H. Tettelin, R. Rappuoli, and E. R. Moxon. 2000. Repeat-associated phase variable genes in the complete genome sequence of Neisseria meningitidis strain MC58. Mol. Microbiol. 37:207-215. [DOI] [PubMed] [Google Scholar]
- 40.Schagger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulphate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 41.Schirle, M., M. A. Heurtier, and B. Kuster. 2003. Profiling core proteomes of human cell lines by one-dimensional PAGE and liquid chromatography-tandem mass spectrometry. Mol. Cell Proteomics 2:1297-1305. [DOI] [PubMed] [Google Scholar]
- 42.Schryvers, A. B., and I. Stojiljkovic. 1999. Iron acquisition systems in the pathogenic Neisseria. Mol. Microbiol. 32:1117-1123. [DOI] [PubMed] [Google Scholar]
- 43.Shevchenko, A., O. N. Jensen, A. V. Podtelejnikov, F. Sagliocco, M. Wilm, O. Vorm, P. Mortensen, A. Shevchenko, H. Boucherie, and M. Mann. 1996. Linking genome and proteome by mass spectrometry: large-scale identification of yeast proteins from two dimensional gels. Proc. Natl. Acad. Sci. USA 93:14440-14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skipp, P. J., J. Robinson, C. D. O'Connor, and I. N. Clarke. 2005. Shotgun proteomic analysis of Chlamydia trachomatis. Proteomics 5:1558-1573. [DOI] [PubMed] [Google Scholar]
- 45.Smith, J. C., J. G. Northey, J. Garg, R. E. Pearlman, and K. W. Siu. 2005. Robust method for proteome analysis by MS/MS using an entire translated genome: demonstration on the ciliome of Tetrahymena thermophila. J. Proteome Res. 4:909-919. [DOI] [PubMed] [Google Scholar]
- 46.Steeghs, L., H. de Cock, E. Evers, B. Zomer, J. Tommassen, and P. van der Ley. 2001. Outer membrane composition of a lipopolysaccharide deficient Neisseria meningitidis mutant. EMBO J. 20:6937-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steeghs, L., R. den Hartog, A. den Boer, B. Zomer, P. Roholl, and P. van der Ley. 1998. Meningitis bacterium is viable without endotoxin. Nature 392:449-450. [DOI] [PubMed] [Google Scholar]
- 48.Tettelin, H., N. J. Saunders, J. Heidelberg, A. C. Jeffries, K. E. Nelson, J. A. Eisen, K. A. Ketchum, D. W. Hood, J. F. Peden, R. J. Dodson, W. C. Nelson, M. L. Gwinn, R. DeBoy, J. D. Peterson, E. K. Hickey, D. H. Haft, S. L. Salzberg, O. White, R. D. Fleischmann, B. A. Dougherty, T. Mason, A. Ciecko, D. S. Parksey, E. Blair, H. Cittone, E. B. Clark, M. D. Cotton, T. R. Utterback, H. Khouri, H. Y. Qin, J. Vamathevan, J. Gill, V. Scarlato, V. Masignani, M. Pizza, G. Grandi, L. Sun, H. O. Smith, C. M. Fraser, E. R. Moxon, R. Rappuoli, and J. C. Venter. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809-1815. [DOI] [PubMed] [Google Scholar]
- 49.Tinsley, C. R., and J. E. Heckels. 1986. Variation in the expression of pili and outer membrane protein by Neisseria meningitidis during the course of meningococcal infection. J. Gen. Microbiol. 132:2483-2490. [DOI] [PubMed] [Google Scholar]
- 50.Tsai, C. M., and C. E. Frasch. 1980. Chemical analysis of major outer membrane proteins of Neisseria meningitidis: comparison of serotypes 2 and 11. J. Bacteriol. 141:169-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsai, C. M., C. E. Frasch, and L. F. Mocca. 1981. Five structural classes of major outer-membrane proteins in Neisseria meningitidis. J. Bacteriol. 146:69-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Turner, D. P., K. G. Wooldridge, and D. A. Ala'Aldeen. 2002. Autotransported serine protease A of Neisseria meningitidis: an immunogenic, surface-exposed outer membrane and secreted protein. Infect. Immun. 70:4447-4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vaughan, T. E., P. J. Skipp, C. D. O'Connor, M. J. Hudson, R. Vipond, M. J. Elmore, and A. R. Gorringe. 2006. Proteomic analysis of Neisseria lactamica and Neisseria meningitidis outer membrane vesicle vaccine antigens. Vaccine 24:5277-5293. [DOI] [PubMed] [Google Scholar]
- 54.Vipond, C., J. Suker, C. Jones, C. Tang, I. M. Feavers, and J. X. Wheeler. 2006. Proteomic analysis of a meningococcal outer membrane vesicle vaccine prepared from the group B strain NZ98/254. Proteomics 6:4203. [DOI] [PubMed] [Google Scholar]
- 55.Virji, M., K. Makepeace, D. J. P. Ferguson, M. Achtman, and E. R. Moxon. 1993. Meningococcal Opa and Opc proteins: their role in colonization and invasion of human epithelial and endothelial cells. Mol. Microbiol. 10:499-510. [DOI] [PubMed] [Google Scholar]
- 56.Virji, M., K. Makepeace, D. J. P. Ferguson, M. Achtman, J. Sarkari, and E. R. Moxon. 1992. Expression of the Opc protein correlates with invasion of epithelial and endothelial cells by Neisseria meningitidis. Mol. Microbiol. 6:2785-2795. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


