The adipocyte and its secretory products have been implicated in a vast array of physiological processes. Work in many laboratories is focusing on the specific contributions of the adipocyte in the areas of metabolism, inflammation, and cancer in order to gain a comprehensive understanding of the systemic influence of the adipocyte under normal and pathophysiological conditions. Until recently, adipose tissue has been considered to be a mere storage compartment of triglycerides. It is now clear that adipocytes are highly active endocrine cells that play a central role in overall energy homeostasis and are important contributors to some aspects of the immune system. They do so not only by influencing systemic lipid homeostasis but also through the production and release of a host of adipocyte-specific and adipocyte-enriched hormonal factors, cytokines, and extracellular matrix components (commonly referred to as “adipokines”).
Little attention has been given to the role of adipose tissue in infectious disease. However, the strong proinflammatory potential of adipose tissue suggests an important role in the systemic innate immune response. Furthermore, the adipocyte proper serves as an important target for the intracellular parasite Trypanosoma cruzi, the cause of Chagas' disease. In chronic Chagas' disease, adipocytes may represent an important long-term reservoir for parasites from which relapse of infection can occur. In other cases, such as with certain subtypes of adenoviruses, infection with viral particles leads to long-term hyperplasia and hyperproliferation of adipocytes, associating these adenoviral infections with a high propensity for subsequent obesity.
Here, we discuss briefly the systemic contributions of adipose tissue to the inflammatory response to infection and provide a brief overview of infectious agents that have a specific impact on adipose tissue.
Adipose tissue is composed of many different cell types, but adipocytes are among the most predominant cells. Since different adipose tissue depots show distinct gene expression patterns and vary widely in size and proximity to neighboring organs, individual depots could be viewed as “mini-organs.” Despite differences between the different pads, the depots share similarity with respect to their ability to store lipids and secrete adipose tissue-derived hormones. Adipose tissue stores lipid in the form of triglycerides and cholesterol esters within the lipid droplets that represent specialized organelles inside the adipocyte. Since the lipid droplet is such a large component of the adipocyte (>95% of the mass of the adipocyte), changes in the amount of lipid stored within the adipocyte affect fat cell size (ranging from 25 to 250 μm). Due to the enormous size of the lipid droplet within a normal adipocyte, these cells were viewed originally merely as lipid storage cells. However, it is now known that adipose tissue acts as an endocrine organ regulating a variety of physiological functions, placing the study of adipokines, or adipose tissue-derived secretory products, at the forefront of research in the field of adipose tissue biology.
ADIPOSE TISSUE AND ADIPOKINES: AN OVERVIEW
Adipose tissue endocrine function was first appreciated in 1989 with the report that the serine protease adipsin is secreted by the 3T3-L1 adipocytes (26). Since this initial report several additional adipokines have been discovered, including adiponectin (Adn) (115), leptin (141), resistin (121), serum amyloid A3 (SAA3) (70), omentin (139), visfatin (39), and RBP4 (138). These adipokines contribute to the regulation of energy homeostasis through effects on both central and peripheral tissues. Several of these adipokines also contribute to nonmetabolic processes in the body, emphasizing the fact that adipokines participate in the coordination of multiple physiological functions in a variety of tissues. Adn is the only adipokine produced exclusively by the adipocyte. All other known adipokines can also be synthesized by tissues in addition to adipose tissue and/or by cells other than adipocytes.
ADIPOSE TISSUE AND THE INNATE IMMUNE SYSTEM
The cellular composition of adipose tissue is not homogeneous. Indeed, adipose tissue contains many different cell types in addition to the adipocyte, including pericytes, monocytes, macrophages, and cells of the endothelium (endothelial and vascular smooth muscle cells). These additional cell types are collectively referred to as the stromal vascular fraction (SVF) of adipose tissue. Since several populations of cells within the SVF of adipose tissue are cells of the immune system, adipose tissue also plays a critical role in the immune response.
The mechanisms by which adipose tissue contributes to the immune response may be through direct effects of resident immune cells within adipose tissue or through indirect effects whereby adipocytes modulate immune cell function in an endocrine and/or paracrine fashion. Most likely, adipose tissue contributes to the immune response through both of these modalities. However, more data are required to determine the importance of either mechanism during an immune challenge or whether the relative importance varies with the source and type of the challenge.
Immune cells are functionally active within adipose tissue, where they exert potent effects on adipocyte metabolism and endocrine function. In the obese state, there is an increase in macrophage accumulation in adipose tissue which correlates with increased expression of cytokines and chemokines including tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), IL-6, IL-8, monocyte chemoattractant protein 1, and IL-18 (132, 136). The increased expression of inflammatory cytokine in adipose tissue has been implicated in the decreased insulin sensitivity (50), increased lipolysis (38), increased leptin production (125), and decreased Adn secretion by adipocytes (77). The increase in adipose tissue cytokine expression, together with the changes in adipose tissue metabolism and endocrine function, contributes significantly to the pathogenesis of the metabolic syndrome associated with obesity. Thus, immune cells within adipose tissue and the inflammatory cytokines that they produce are active and can significantly alter adipose tissue metabolism and endocrine function. In addition, increases in adipose tissue inflammatory cytokine levels may affect nonadipose tissues either directly or indirectly. Some adipose tissue-derived inflammatory cytokines such as TNF-α are not secreted into the circulation to any significant extent and are only elevated locally; however, increases in adipose tissue expression of TNF-α contribute to changes in whole-body metabolism and the in vivo hormonal milieu (127). A likely explanation for this finding is that the effects of TNF-α on nonadipose tissue are likely mediated by changes in the expression of adipokines such as leptin and Adn. In contrast, cytokines such as IL-6 are secreted by adipose tissue into the circulation. Interestingly, a highly significant fraction of circulating IL-6 is adipose tissue derived (85, 100). Therefore, the production of inflammatory cytokines within adipose tissue and the contribution of adipose tissue-derived cytokines to circulating levels may play a significant role in host defense mechanisms during immune challenges. These observations underscore the potential relationship between adipose tissue-derived immune cells and their contribution to an immune response and how adipocytes contribute to immune cell-stimulated changes in peripheral tissues through the secretion of adipokines. However, the teleological reasons why such a tight connection between the immune system and energy stores exists are unknown. Why are immune cells within adipose tissue and how does the presence of adipose tissue modulate immune cell function during an acute immune challenge? Several laboratories are starting to explore these questions. Recent evidence derived from coculture experiments demonstrates the relationships between adipocytes and macrophages whereby secretory products from adipocytes amplify macrophage inflammatory cytokine expression in vitro (13). Similar findings are reported in vivo, where inducible ablation of adipocytes blunts the lipopolysaccharide (LPS)-stimulated rise in systemic levels of IL-6 in mice (100). The factors secreted by adipocytes capable of stimulating or amplifying inflammatory cytokine production by resident macrophages within adipose tissue may include acute-phase reactants such as SAA3 (70). Treatment of monocyte cell lines in addition to cultured SVF cells with SAA3 results in a marked induction of inflammatory mediators such as IL-6, IL-8, and TNF-α. It is possible that the acute-phase reactants synthesized by adipocytes may contribute to the synergy observed between adipocytes and the immune cells (70). Additional acute phase reactants are produced by adipocytes that include α1 acid glycoprotein, the CRP relative pentraxin-3, and a number of lipocalins such as 24p3 and retinol-binding protein 4 (Rbp4).
OBESITY AND SEPSIS
It is well established that obesity is associated with increased risks of morbidity and mortality (1, 12, 80). Obesity results in increased admissions into hospital critical care units. Interestingly, the mortality after blunt trauma is increased eightfold in morbidly obese patients (80). Intubated patients who are obese have an increased risk of aspiration pneumonia than nonobese patients. This may be due to several factors, such as higher intra-abdominal pressures and an increased incidence of gastric reflux and higher volumes of gastric fluid with lower pH in obese individuals (80). Moreover, a larger number of skin punctures is often required to gain vascular access in obese patients, thus increasing the risks of catheter-related infections and thromboses (18). Postoperatively, the risk of developing a surgical wound infection is greatly increased in obese patients, especially with abdominal surgery, which independently carries an increased risk of postoperative infections (1, 67).
In view of the role of adipose tissue as an endocrine organ and its huge potential to secrete several pro- and anti-inflammatory mediators such as cytokines, hormones, and peptides, it is likely that under conditions when adipose tissue can contribute between 30 and 50% of total body weight, the increase in morbidity and mortality in septic obese patients could be attributed to this excess adipose tissue. Adipocytes express many of the known Toll-like receptors (TLRs). In fact, we found that that adipocytes are highly responsive to endotoxin due to the high-level expression of TLR-4 (69) and respond by inducing high levels of proinflammatory cytokines at concentrations of endotoxin that reflect a sensitivity to these bacterial cell wall components comparable to or higher than what can be observed in macrophages. In the same study, we reported the induction of TLR-2 in adipocytes, which confers a high degree of sensitivity to fungal cell wall components.
The role of adipose tissue in the pathogenesis of infection was underscored by our recent observations when we generated an inducible “fatless” model system, the FAT-ATTAC (for fat apoptosis through targeted activation of caspase 8) mouse (100). In this model, adipocytes can be inactivated in a triggered fashion. This allows the assessment of a complete absence of functional adipocytes prior to the onset of conventionally observed secondary effects associated with chronic lipodystrophy. In the absence of adipocytes, the “septic response” to LPS injections was dramatically reduced as judged by a decrease in the systemic IL-6 and SAA3 levels.
Somatostatin, a neuroendocrine secretory product, has been described in immune cells after activation by the inflammatory process and is induced by administration of IL-1β and TNF-α (113, 114). Somatostatin inhibits the secretion of several hormones in the gastrointestinal tract, including insulin and glucagon (101). Cytokines and LPS induce somatostatin transcription in human adipose tissue (116). Increased somatostatin transcription also occurs when adipose tissue is cocultured with Escherichia coli or activated macrophages. In patients with severe bacterial infections, the increase in somatostatin mRNA and protein expression is observed in visceral fat depots but not in subcutaneous fat nor in depots from uninfected patients. This increase is inhibited in the presence of dexamethasone (116).
Adipose tissue is also an important extrathyroidal source of inflammation- or sepsis-induced calcitonin and calcitonin precursors. During sepsis there is a thousandfold increase in the expression of calcitonin and procalcitonin that can be attributed to adipose tissue (71-73). This increase directly correlates with disease severity and mortality and occurs both in subcutaneous and in omental fat depots. The addition of endotoxin to cultured human adipose tissue, as well as the administration of IL-1β and TNF-α, causes an increase in calcitonin and its precursors; the effects of IL-1β are reversible after treatment with IFN-γ (71). These data demonstrate a possible role for calcitonin in the increased morbidity and mortality observed in morbidly obese patients in the critical care setting. In this regard supraphysiologic doses of procalcitonin increased mortality (94), and peak levels during infection can serve as early markers of sepsis and an indicator of mortality (120, 133). In addition, procalcitonin has been shown to stimulate inducible nitric oxide synthase (iNOS) transcription in vascular smooth muscle cells (48). Interestingly, anti-procalcitonin serum reversed the deleterious effects and increases survival in sepsis (94). During sepsis, adipose tissue also modulates the increased secretion of adrenomedullin and of the calcitonin gene-related peptide, both of which are potent vasodilators and modulators of nitric oxide production. Notably, these proteins induce insulin resistance during sepsis (72, 73).
In patients with septic shock due to fulminate cellulitis, there was a compartmentalization of increased iNOS activity and IL-1β to inflamed regions of fat, muscle, and arteries that did not occur in skin, the primary site of infection. Interestingly, the increase in TNF-α expression was not altered in fat tissue in those cases (5). The expression of iNOS was inversely related to mean arterial pressure and to systemic vascular resistance, suggesting a role in cardiovascular failure in septic shock (5).
Sepsis has also been associated with disturbances in lipid metabolism resulting in hypertriglyceridemia and lipid accumulation in the liver. In animal models, it was demonstrated that E. coli-induced sepsis resulted in a significant decrease in lipoprotein lipase (LPL), leading to a marked decrease in clearance of triglycerides and free fatty acids in cardiac tissue, adipose tissue, and muscle (64, 65). This was associated with an increase in de novo hepatic lipogenesis (65, 131). The administration of LPS and many cytokines to animals rapidly increased serum triglyceride levels which persisted (65). The alterations in lipoprotein metabolism differed depending on the amount of endotoxin used (58). At low doses of LPS or cytokines, there was an increase in de novo hepatic fatty acid synthesis, particularly short-chain fatty acids (131), lipolysis of adipose tissue, and suppression of fatty acid oxidation. Higher doses of LPS induced a decrease in LPL activity in muscle and adipose tissue, thereby decreasing the clearance of very-low-density lipoproteins. LPS and cytokines cause a decrease in apoE mRNA; apoE is believed to be critical in the clearance of triglyceride-rich lipoproteins (58). LPS decreased fatty acid uptake in cardiac and skeletal muscle and adipose tissue (84), and IL-4 reversed some of the actions of these cytokines and of LPS. Interestingly, the response to LPS and cytokines was significantly higher in subcutaneous adipose tissue than in visceral adipose tissue.
Bacterial infection induces fatty acid synthesis, increases lipolysis, generates fatty acids from adipose tissue, and reduces fatty acid oxidation and ketogenesis in the liver. The increased fatty acids are re-esterified in the liver a process mediated by liver acyl-coenzyme A synthetase (ACS) activity. ACS is essential in the regulation of fatty acid metabolism. It allows the catalysis of long-chain fatty acid to acyl-CoA esters, which can be utilized in the synthesis of cellular lipids or in β-oxidation (84). ACS is located in the microsomes, mitochondria, and peroxisomes of the liver, as well as in microsomes of adipose tissue and mitochondria of heart and muscle. The administration of LPS and cytokines caused a marked decrease in the transcription of ACS in the liver, adipose tissue, and cardiac and skeletal muscle tissue (84). In the liver, although mitochondrial activity was reduced, there was an increase in the microsomal activity (84). Mitochondrial ACS regulates activation of fatty acids for oxidation and ketone body production as sources of energy, and microsomal ACS is believed to regulate the re-esterification of fatty acids for triglyceride synthesis (59). Importantly, the administration of insulin reversed the decrease in LPL observed in sepsis. In rats infected with E. coli, isolated adipocytes demonstrated a sustained decrease in LPL activity. However, after overnight treatment with insulin, there was a significant increase in LPL synthesis and activity. Hence, the sepsis-induced decrease in adipose LPL is not a direct consequence of insulin resistance; rather, insulin stimulates LPL synthesis in septic rats (103).
INSULIN RESISTANCE AND GLUCOSE METABOLISM IN SEPSIS
Glucose metabolism is markedly altered during bacterial sepsis, and hyperglycemia in the face of hyperinsulinemia is well documented in situations of stress such as during systemic infection and shock. The diabetic state worsens during infection, and this is believed to be due, in part to the increase in certain hormones, such as cortisol, which occur during stress, but the mechanism for this apparent resistance to insulin is largely unknown. During sepsis, there is an alteration of insulin receptors both in quantity and in affinity (51). In a study of induced sepsis in rats, no difference was demonstrated in insulin binding in septic versus control rats despite a significant increase of plasma glucose levels. This observation suggests no change in quantity or affinity of insulin receptors in that model. There was, however, a marked difference in the uptake of glucose by adipocytes in the presence of insulin in a dose-dependent manner, demonstrating a significant decrease in responsiveness to insulin (51). The study by Igarashi et al. (51) demonstrated that in the rat sepsis model, hyperglycemia in sepsis may be partly due to postreceptor defects in insulin-mediated glucose transport by adipocytes. High levels of circulating glucose may be proinflammatory since they can lead to leukocyte rolling and adherence and can be associated with the production of free oxygen radicals in aortic endothelial cells (91, 92), as well as induce potent proinflammatory responses in adipocytes (68). Importantly, in critically ill patients the administration of exogenous insulin has a significant effect on survival (129). Thus, the systemic anti-inflammatory effects of insulin during sepsis are increasingly appreciated and are now used in septic patients. Beyond its effects contributing to a reversal of sepsis-induced alterations in lipid metabolism and hyperglycemia, insulin may in addition suppress the expression of NF-κB, thereby reducing the production of cytokines such as TNF-α (91). A more detailed view of the anti-inflammatory properties of insulin has been discussed by Dandona et al. (27).
OBESITY AND ANTIBIOTIC PHARMACOKINETICS
The physiologic changes associated with obesity also influence the distribution, clearance, and metabolism of drugs. Underlying sepsis may also complicate the pharmacokinetics of drugs, including antibiotics (20, 54, 80, 112, 140). The increased levels of lipoproteins, cholesterol, and free fatty acids that competitively bind serum albumin may inhibit the protein binding of certain drugs; furthermore, the lipophilicity of drugs largely determines their volume of distribution (80). In septic shock, there have been several reports of impaired penetration of β-lactam antibiotics at sites of infection (54, 140). The levels of these antibiotics in subcutaneous adipose tissue were significantly less than the levels found in plasma (20, 54, 112). Since infections are usually localized to the interstitial fluid of tissues rather than in plasma, the decrease in penetration may contribute in part to therapeutic failures during septic shock in the face of inhibitory plasma concentrations (54). This phenomenon was recently observed with the antibiotic linezolid, where patients with septic shock had lower concentrations of the drug than healthy volunteers (20). The fluoroquinolone levofloxacin was found to achieve similar concentrations in inflamed and healthy subcutaneous adipose tissue; thus, not all antibiotics are affected by inflammation caused by infection (10). In summary, the available data indicate a large variability of drug concentration in inflamed tissue from individual to individual during septic conditions and poses a major challenge in determining the appropriate treatment doses.
ADIPOKINES LEPTIN AND ADIPONECTIN: CRITICAL MEDIATORS IN THE INFLAMMATORY RESPONSE DURING INFECTION?
Leptin and Adn are two adipokines important in the modulation of the host response to infection. Leptin, the 16-kDa nonglycosylated protein product of the obese (ob) gene (141), is a pleiotropic hormone-cytokine synthesized mainly in adipocytes (78). Leptin and leptin receptors have been identified in a variety of tissues and organs (7, 19, 47, 82). The biological activities of leptin are mediated through the interaction with its specific membrane receptor (Ob-R), which has sequence homology to members of the class I cytokine receptor (gp130) superfamily (124). Leptin is released into circulation, and its levels correlate with total body fat mass (2). During malnutrition or starvation, circulating leptin levels decline and serve as a signal to the host to conserve energy by reducing energy metabolism (3). Although leptin has been more strongly associated with an increase in metabolic rate and reduction of adipose mass by suppressing appetite and stimulating the sympathetic nervous system (16, 17, 22, 23), it is also implicated in negative feedback of the hypothalamic-pituitary-adrenal axis. Leptin receptors are found in the central nervous system and in peripheral tissues. Accordingly, the physiological role of leptin is not solely restricted to the regulation of food intake and energy expenditure. Leptin plays a role in hematopoietic differentiation, including lymphoid cells of the immune system, and can be an inducer of the host inflammatory response (11, 40, 74, 75, 81, 99, 107, 108).
Obese leptin-deficient ob/ob mice and db/db mice display immune dysfunction and lymphoid organ atrophy, and thus they have reduced levels of T and B cells (75). Missense mutations in humans leading to leptin deficiency also result in immune system dysfunction (99). Leptin induces the proliferation, differentiation, and functional activation of hematopoietic cells. Hence, bone marrow adipocytes may play an important role in this process. Leptin modulates the proliferation and activation of peripheral T lymphocytes in mice and in humans (75, 81) and can enhance the production of cytokines (18, 19). In addition, leptin modulates monocyte-macrophage function and regulates proinflammatory responses (40, 107). Leptin increases Th1-dependent immunostimulation and autoimmune diseases such as experimental autoimmune encephalomyelitis in SJL mice. The deficiency of leptin signaling decreases both humoral and cellular immune responses in arthritis (21). It also modulates T-cell mediated inflammation, enhances proliferation, and IL-2 production of native T cells and promotes the Th1 phenotype by increasing IFN-γ and inhibiting IL-4 production from CD4 cells (75). Leptin enhances the synthesis of proinflammatory cytokines by cultured macrophages after treatment with LPS (74), and similar observations have been made in vivo in rodents. Therefore, the involvement of leptin in the upregulation of the inflammatory response provides a common pathogenic mechanism for several of the major complications of obesity.
Leptin-deficient mice have an impaired host defense in a bacterial pneumonia model that may be due to a defect in alveolar macrophage phagocytosis and leukotriene synthesis (79). The role of leptin in the host defense against gram-negative pneumonia was studied by comparing the responses of wild-type and leptin-deficient mice to an intratracheal challenge with Klebsiella pneumoniae. In this model, there was an increase in systemic and pulmonary leptin levels that was deemed critical for host defense. The absence of leptin triggered greater mortality and reduced bacterial clearance from the lungs of the leptin-deficient mice compared to wild-type mice. Interestingly, no differences were observed between wild-type and leptin-deficient mice in lung homogenates with respect to TNF-α, IL-12, or macrophage inflammatory protein 2 levels after infection; however, there was a reduction in leukotriene production in leptin-deficient mice. These data strongly suggested that leukotrienes are important mediators of leptin effects on macrophage phagocytosis in mice. The absence of leptin generated marked impairment of polymorphonuclear neutrophil phagocytosis of bacteria (86). This phenomenon was reversible by leptin replacement in the leptin-deficient mice. Recent studies also indicate that leptin has a role in the early immune response during pulmonary infection caused by Mycobacterium tuberculosis in mice by mediating an effective IFN-γ-driven Th1 response with adequate lymphocyte trafficking and granuloma formation (135). Bleau et al. (14) reported that an isolate of Lactobacillus acidophilus decreased both leptin production and leptin-mediated immunostimulation, leading to a lower Th1 response by SJL adipocytes, and suggested that this was the mechanism by which this strain of Lactobacillus had a protective role in intestinal inflammatory diseases in their model.
The downstream effects from the leptin receptor in immune cells are mediated by activation of the JAK-STAT signaling pathway in peripheral blood mononuclear cells (106). After leptin stimulation, JAK activity was increased and STAT-3 is tyrosine phosphorylated, dimerized, and translocated to the nucleus to activate gene expression. STAT-3 was also associated with tyrosine-phosphorylated src substrate Sam68 in response to leptin. Martin-Romero et al. (81) demonstrated that leptin stimulation of peripheral blood mononuclear cells promotes the tyrosine phosphorylation of the intracellular domain of the leptin receptor, IRS-1, and Sam68 via JAK activation. The substrates IRS-1 and Sam68 associate with p85, providing a molecular mechanism for phosphatidylinositol 3-kinase activation. Leptin also activates various mitogen-activated protein kinase pathways (57, 122).
Plasma levels of leptin are elevated during inflammation or infection (22). Induced peritonitis in rodents leads to an increase in plasma leptin concentrations and in transcription of leptin in adipocytes (17, 22). In patients that had been septic for ≥14 days, there was no correlation between sepsis and serum leptin concentrations (22). However, during acute bacterial sepsis, there appeared to be a significant increase in plasma leptin levels associated with a marked increase in cortisol level and IL-6. In these patients there was a complete blunting of the circadian pattern of leptin release. Interestingly, the pattern was reversed with lower levels of leptin at night and higher levels during the day (17). These observations suggest that leptin may contribute to the anorexia observed during sepsis. In addition, leptin can directly suppress adrenal function, thus providing interesting implications for the mechanism of adrenal insufficiency observed during sepsis. In the present study, patients with higher levels of circulating leptin had a better survival rate compared to nonsurvivors, suggesting that leptin could serve as a marker for acute stress-mediated response. Although leptin administration in vivo plays a protective role in murine pulmonary antibacterial host defense (79), in humans the role of leptin and microbial infection remains unclear. However, recent developments suggest that studies exploring the role of leptin as both a diagnostic marker and a therapeutic agent to augment the immune response during sepsis are warranted.
Adn is a secretory protein synthesized exclusively by adipocytes. It serves as an anti-inflammatory cytokine and antagonizes the effects of TNF-α. When the plasma levels of Adn were measured in an animal model of polymicrobial sepsis, there was a gradual decrease in Adn levels to ∼50% at 24 h. Moreover, there was an inverse relationship with levels of circulating TNF-α (126). Adn was also demonstrated to affect LPS activity in vitro. When Adn was incubated with LPS, there was a significant dose-dependent decrease in the activity of LPS. Western blot analysis demonstrated evidence of binding of Adn to LPS, suggesting that Adn may have a neutralizing effect on LPS, although this has not been proven in vivo. It is not clear whether Adn itself has anti-inflammatory properties or whether indirect mechanisms are at work. However, Adn production by adipose tissue can be inhibited by systemic inflammation, at least under some circumstances. Adn production by cultured adipocytes was demonstrated to be inhibited by inflammatory cytokines (37, 105), and this inhibition may be mediated in part by NF-κB signaling. In cultured adipocytes and in obese diabetic mice, inhibition of adipocyte inflammatory NF-κB signaling by an IκB kinase inhibitor resulted in a reduction of cytokine levels and increased plasma levels of Adn (55). Thus, IκB kinase inhibition leads to increased plasma Adn levels and an improvement in systemic insulin sensitivity. In contrast, Keller et al. did not observe an effect on Adn levels after LPS administration to humans (56).
The anti-inflammatory action of Adn may be mediated by one of its principal signaling targets, the AMP-activated protein kinase (AMPK) (137). There is evidence that the truncated bacterial form of an Adn has anti-inflammatory effects on endothelium via AMPK-mediated modulation of NF-κB and Akt/PKB (95, 97, 98). However, whether such a trimeric globular form exists in vivo in not clear, thus making the interpretation of these results for an in vivo situation difficult. Some of the proven antiatheromatous effects of Adn may be mediated by anti-inflammatory activities acting directly on the vasculature.
Adn has an important role in protecting against cardiac hypertrophy in cardiac overload states such as hypertension, hypertrophic cardiomyopathy, and ischemic heart disease. In mice, this has been supported by data demonstrating that Adn protects against overload-induced and adrenergically induced cardiac myocyte hypertrophy by inhibiting hypertrophic signals via AMPK (117). Interestingly, Adn may represent the link between obesity (associated with decreased Adn levels) and cardiac risk by protecting against inflammation-induced cardiovascular insufficiency and direct myocardial insults.
HIV INFECTION AND ADIPOCYTES
Patients infected with human immunodeficiency virus (HIV)/AIDS undergo many metabolic abnormalities, including dyslipidemia, insulin resistance, fat loss, or lipoatrophy and fat accumulation. Adipocytes play an important role in HIV infection since significant changes in adipose tissue morphology and metabolism are observed in HIV-infected individuals. While many authors demonstrate that HIV lipodystrophy is associated with peripheral adipose tissue loss with a concomitant increase in visceral adiposity (43), others have demonstrated that both subcutaneous and central depots are affected and undergo reduction (6). The underlying mechanisms for this phenomenon remain poorly understood. This lipodystrophy syndrome particularly prevalent in patients on highly active antiretroviral therapies is associated with other metabolic complications, including insulin resistance, dyslipidemia, cholesterol, and fat redistribution (90). Most studies have suggested that the lipodystrophy is associated with the use of two major classes of antiretroviral drugs, nucleoside reverse transcriptase inhibitors (NRTIs) and protease inhibitors (PIs) (45). Subcutaneous fat cell biopsies of HIV-infected patients treated with PIs demonstrated increased adipocyte cell death in the subcutaneous fat compartments (34), whereas others have found little evidence for apoptosis (88).
Adipocytes could be a direct target for HIV infection. Hazan et al. (46) demonstrated the receptors for HIV entry, such as CD4, CXCR4, and CCR5, were expressed in vitro on preadipocytes and adipocytes by reverse transcription-PCR and immunocytochemical experiments. In vivo expression of these receptors was also demonstrated on sections of human white adipose tissue by immunohistochemistry. However, these authors concluded that in vitro infection of adipose tissue with the virus was not achievable because the level of expression of these HIV receptors did not permit entry into adipose cells in the biopsies tested (87). Nevertheless, Maurin et al. demonstrated HIV receptor expression in freshly isolated preadipocytes and in mature adipocytes from subcutaneous fat depots (83). These authors also demonstrated HIV infection of fat cells by observing HIV-1 transcriptional activity in the fat cells and provided evidence for adipocyte infection with HIV by showing significantly increased Gag p24 antigen expression upon stimulation of these cells with proinflammatory cytokines, such as TNF-α or IL-1β. The results of that study indicate that HIV-1 indeed infects human adipose cells in vitro and further suggest that the initial infection may be overcome by treatment with proinflammatory cytokine stimulation.
Altered fat differentiation and morphology has been described in HIV patients (52), as well as increased levels of collagen fibers and vessel density. Leptin levels were significantly higher in HIV-infected patients with lipodystrophy, and these levels were negatively correlated with insulin resistance (61). Adn levels were reported to be lower in HIV-infected patients with lipodystrophy and positively correlated with insulin resistance (89). Increased levels of TNF-α and IL-6 during HIV infection may directly affect the insulin signaling pathways and induce insulin resistance. Moreover, the lipolytic effects of these cytokines may increase free fatty acids and may also cause insulin resistance through lipotoxic effects in muscle and liver (119). The impact of PIs and NRTIs on adipose cell functions and adipokine production in vivo and in vitro has been reviewed elsewhere (62).
Adipocyte differentiation involves the sequential activation of transcription factors which regulate the expression of adipocyte specific markers. CCAAT-enhancer binding protein β (C/EBPβ), peroxisome proliferator activated receptor γ (PPARγ), and C/EBPα are major transcription factors that become activated during the differentiation of adipocytes. Differentiation is enhanced by the transcription factor sterol regulatory element binding protein 1 (SREBP1), which activates PPARγ (36, 104). Bastard et al. demonstrated reduced mRNA expression of the adipogenic differentiation factors C/EBPβ and C/EBPα, PPARγ, as well as the 1c isoform of SREBP1, in the subcutaneous fat of individuals with HIV lipodystrophy treated with PIs compared to healthy subjects (9). PPARγ activates C/EBPα which, in turn, enhances and maintains PPARγ expression, allowing the expression of adipocyte specific genes and cell differentiation in central fat depots, in which SREBP-1 protein levels are also increased (9). PI-induced alterations of SREBP-1 expression may contribute to the changes in fat distribution in HIV-infected individuals, similar to the changes that can be seen in mice with altered SREBP1c levels (118). PIs (i.e., nelfinavir, ritonavir, and lopinavir) and some NRTIs (i.e., stavudine and zidovudine) induce the expression and secretion of IL-6 and IL-1β in 3T3-L1 cell lines, suggesting that these drugs can modulate the complement of secretory proteins regardless of the differentiation status (62). This includes altered expression of TNF-α, IL-6, Adn, and leptin, which has also been observed in the adipose tissue of HIV-infected patients, leading to increased serum levels of TNF-α and IL-6 levels in lipodystrophic HIV patients (9, 52, 53, 93).
INFECTIOUS ETIOLOGY OF OBESITY
Although the etiology of obesity is multifactorial, there has been an increasing interest in the fascinating possibility that infection with specific pathogens may lead to increased adiposity (28-30, 42). Scrapie, a prion neurodegenerative disease of goats and sheep with a prolonged incubation, was also reported to cause obesity in mice. The ability of scrapie to cause obesity appears to be strain specific. The observations of Kim et al. (60) suggest that the mechanism is an alteration of the hypothalamic-pituitary-adrenal axis while others have suggested that alterations in the glucose transporter (GLUT-1) may be important in the pathogenesis of obesity in this model (130).
An underlying viral basis was first suggested by Lyons et al. in 1982 (76), who reported that canine distemper virus (CDV) caused obesity in mice. CDV causes respiratory, gastrointestinal, and central nervous system (CNS) diseases in dogs and other mammals. This virus invades the CNS replicating in neurons and glial cells of the white matter and causes an encephalomyelitis. Mice infected with CDV experience an increase in body weight, fat cell size, and fat cell number. The mechanism for this observation was believed to be the result of virus-induced hypothalamic damage. However, it has also been suggested that that the virus-induced downregulation of leptin receptor expression in the hypothalamus of CDV-infected obese mice may be the cause of the obesity. Another virus, Rous-associated virus-7 (RAV-7), an avian retrovirus was reported to cause stunted growth, obesity and hyperlipidemia in chickens. The infected chickens develop fatty enlarged livers, anemia and immunodepression. Carter et al. (24) suggested that a reduction in thyroid hormone level in RAV-7-infected chickens was the cause of the obesity and hyperlipidemia.
Borna disease virus (BDV) which was also reported to cause obesity infects many birds and mammals and persists in the nervous system. BDV antibodies were detected in horses in many parts of the world. Gosztonyi and Ludwig demonstrated that this virus caused obesity in rats and that this was associated with inflammation of the hypothalamus, pancreatic hyperplasia, and elevated levels of triglycerides and glucose (41). Viral antigen expression was noted in several areas of the brain including the hypothalamus. Thus, infection with BDV could result in neuro-endocrine dysregulation leading to obesity. Interestingly, BDV-specific antigens and BDV-RNA have been demonstrated in human brain. This virus has also been demonstrated to be associated with a variety of psychiatric disorders.
The first virus that was reported to be associated with human obesity was the avian adenovirus SMAM-1. Dhurandhar et al. found antibodies to this avian adenovirus in obese individuals in India (32). However, since this virus was not thought to infect humans, the observation suggested cross-reactivity to an antigenically similar human virus. The same authors first reported that Ad-36 caused obesity in animals (31). In small animal studies, they demonstrated the presence Ad-36 DNA in adipose tissue of Ad-36-induced obese animals. Furthermore, these authors demonstrated the potential of Ad-36 in promoting obesity in nonhuman primates (33).
Human adenoviruses commonly cause infections of the respiratory and intestinal tract and the eye. Thus, antibody titers to adenoviruses are common in the population. There are 50 adenoviruses that are placed into six major subgroups (A to F) with specific serotypes. Ad-36, implicated in human obesity is part of group D, serotype 36. Ad-36 does not ordinarily cross-react with antibodies to other human adenoviruses. It has an unusual affinity for adipose tissue, and the amount of Ad-36 DNA in visceral adipose tissue correlates with the fat depot weight. Approximately 30% of the obese and 11% of the nonobese individuals demonstrated antibodies to Ad-36 (30). Although the precise mechanism by which this virus promotes obesity is unclear, it has been reported that Ad-36 upregulates preadipocyte differentiation in culture. The demonstration that adipocyte differentiation, leptin production, and glucose metabolism is modulated by Ad-36 is of interest, but it remains to be conclusively proven that this virus is adipogenic in humans. For example, the recent report by Whigham et al. (134) demonstrated that Ad-31 caused increased differentiation of 3T3-L1 cells, but this did not correlate with obesity in chickens. Furthermore, there was no correlation between the development of obesity in chickens induced by Ad-37 and human obesity. The association of adenoviral infection with obesity is highly specific for Ad-36, which is at this time the only human adenovirus implicated with human obesity.
Finally, recent reports suggest an association of Chlamydia pneumoniae infection with human obesity (30). This is of interest in view of the many reports suggesting that this organism might be associated with the pathogenesis of arteriosclerosis and coronary heart disease. Research into the relationship between obesity and infection has only begun to be investigated and more data are needed to establish definite cause and effect relationships.
TRYPANOSOMA CRUZI INFECTION AND ADIPOSE TISSUE
Parasitic diseases, caused by both nematodes and protozoa, have been reported to be associated with nutritional deficiencies, wasting, and diabetes. Chagas' disease, caused by the parasite Trypanosoma cruzi is endemic in Latin America. In recent years, the influx of immigrants from these countries into North America and Europe has led to an increase in the diagnosis of clinical Chagas' disease in the United States, as well as the recognition of blood transfusion-induced Chagas' disease in areas where this disease is not endemic (66). Reactivation of Chagas' disease may present as an opportunistic infection in individuals with AIDS/HIV (128) and in those undergoing immunosuppressive therapy for malignancies and in organ transplantation (8). When individuals with chronic and/or asymptomatic T. cruzi infection acquire HIV infection the reactivation of this infection (111) may present as a necrotizing encephalitis and/or acute myocarditis (63, 102, 109, 110). Acute chagasic myocarditis may be the sole manifestation of reactivation, or it may accompany encephalitis.
For many years an association between human T. cruzi infection and obesity and diabetes has been suspected, and there has been general belief that the incidence of diabetes is greater in the chagasic population. Indeed, in recent years there have been several reports indicating that diabetes is more common in this population (35, 44, 96). One such study demonstrated a significant reduction in insulin among chronically infected individuals (44). Interestingly, previous studies from our laboratory demonstrated that when mice with chemical-induced diabetes or diabetic db/db mice were infected with T. cruzi, they had a higher parasitemia and mortality (123). The underlying mechanisms of these observations remain unknown. Interestingly, chemically induced diabetic mice infected with the causative agent of African trypanosomiasis, T. brucei developed higher parasitemia and increased mortality compared to infected nondiabetic mice (4).
As noted above, the adipocyte has been identified as an important contributor in the pathogenesis of diabetes, obesity, and the metabolic syndrome, but its relationship to the pathogenesis of T. cruzi infection has only recently been explored. It has been suggested that adipose tissue in Chagas' disease may serve as one of the reservoirs for the parasite from which recrudescence may occur during immune suppression. However, since no systematic approach has been undertaken to more precisely define the role of the adipocyte in the normal and diabetic state during infection with T. cruzi, we examined the direct effects of T. cruzi infection on adipocytes in vitro and in vivo.
Mice were infected with the trypomastigotes of T. cruzi. To determine the metabolic consequences of infection, basal glucose levels as well as insulin sensitivity were monitored by means of an oral glucose tolerance test prior to infection, during the acute phase of infection, as well as during the chronic stage. We observed that acute infection was often associated with severe hypoglycemia which correlated with mortality (25), an observation made by others as well (49). As noted above, the metabolic response to bacterial sepsis usually includes hyperglycemia with insulin resistance, profound negative nitrogen balance, and diversion of protein from skeletal muscle to splanchnic tissues. The response to T. cruzi infection, however, differs from that observed in most studies of bacterial sepsis. One possible mechanism is an effect on glucose metabolism due to invasion of the liver by the parasite. However, the precise underlying reasons for hypoglycemia during acute T. cruzi infection are not known. Surprisingly, during acute infection, glucose levels in the infected mice at all stages remained below the levels measured for the control animals. Even though the baseline glucose levels in the infected animals were lower, the oral glucose tolerance test indicated relatively normal ability to clear the ingested glucose despite the high degree of inflammation associated with this infection. The fasting glucose levels and survival rates of infected mice and uninfected mice were compared, and a strong correlation between the relative reduction in glucose levels and mortality was observed (25).
The level of Adn, the adipokine that was discussed above, is significantly decreased during T. cruzi infection in mice. Decreased levels of Adn are usually associated with insulin resistance, hyperglycemia, and obesity. In addition, decreased levels of Adn are observed with increased systemic inflammation associated with cardiovascular disease. However, acute inflammation triggered by endotoxemia does not have an effect on Adn levels (56). Adn levels were reduced during acute T. cruzi infection, suggesting that the proinflammatory component of the infection is the main driving force for the expression of Adn. The hypoglycemia cannot be readily explained by changes in Adn. This is an example of a physiologically relevant condition that combines hypoglycemia and normal glucose tolerance with significantly reduced Adn levels. It is noteworthy that individuals with malaria infection have increased levels of Adn (15).
The decreased insulin levels observed 30 days postinfection in the mouse model of T. cruzi infection are consistent with a physiological response to the very low glucose levels during that time. It is unlikely that this is a reflection of pathological changes at the level of the pancreatic β cells, since the morphology is normal at day 30 postinfection and insulin levels revert to normal levels at later stages. In addition, leptin levels were significantly reduced in infected mice compared to controls. Resistin levels, another fat cell-specific secretory factor with insulin-desensitizing properties, were not affected by infection. Levels of plasminogen activator inhibitor 1, which is also prominently expressed in adipocytes, are completely unaffected by infection. In contrast, proinflammatory markers such as IL-6, MCP-1, and TNF-α were markedly elevated in infected mice in response to the high parasite load on day during the acute infection. The significant decrease in leptin levels was initially surprising since the infected mice gained more weight than the control mice. However, magnetic resonance imaging studies revealed that there were significantly reduced levels of adipose tissue during the acute phase of the infection. Interestingly, the decrease in adipose tissue persisted even at later stages during chronic infection and was primarily caused by a decrease in abdominal adipose tissue. Mice that had marked right ventricular dilation had an even greater loss of fat depots. The weight gain in infected mice appeared to be related to edema, which may have been the consequence of right-sided heart failure (25).
Since the plasma levels of Adn were reduced as a result of T. cruzi infection, we wanted to determine whether the parasite has the potential to actually invade adipocytes in vivo. Real-time PCR data demonstrated that adipose tissue is a relevant target tissue, harboring large numbers of parasites. Particularly noteworthy is that at 300 days postinfection, a comparable number of parasites were present in adipose and heart tissue, indicating that adipose tissue is a reservoir tissue for these parasites. We observed that adipocytes were readily infected in vivo. Since there were numerous parasites in adipose tissue, we suspected that the massive local parasite count should be responsible for a considerable influx of macrophages. We wanted to determine whether the high propensity to harbor and propagate T. cruzi in adipocytes also has an effect on macrophage levels. Therefore, white adipose tissue was isolated from two different fat depots of infected mice. As judged by an immunohistological stain for the macrophage-specific marker F4/80, there was a marked increase of F4/80-positive cells in white adipose tissue during the acute infection. Even more pronounced changes were observed in brown adipose tissue. This persisted even at 300 days postinfection. The parasites were located within the adipocytes of brown fat, which is characterized by multiple smaller intracellular lipid droplets compared to the densely packed white adipocytes that contain a single large lipid droplet. During the chronic stage of infection, there was a reduction in the number of macrophages compared to the acute infection (25).
The reduced levels of Adn in plasma in T. cruzi infection (25) suggest that infection of adipocytes may also have consequences on other proteins synthesized in adipose tissue. Therefore, immunoblot analysis was performed for a number of proteins that are expressed in adipose tissue. Consistent with the reduction of plasma Adn, the levels of Adn in adipose tissue were also reduced during acute infection in a number of fat pads, particularly in perirenal and visceral adipose pads, both of which are important systemic sources of Adn, whereas the levels in brown adipose tissue were not affected. We extended the analysis of protein expression on brown and perirenal adipose tissue 30 days postinfection to a number of additional inflammatory markers. The acute-phase reactants α-1 acid glycoprotein and SAA3, which are expressed in adipocytes, were upregulated. The expression of TNF-α, IFN-γ, and IL-1β that were also increased acutely, and these changes persistent even to 90 days postinfection. The 3T3-L1 cells, widely used as a model for primary adipocytes, were infected with T. cruzi. Infection triggered a reduction of Adn levels, while the levels of Toll-like receptor 2 (TLR-2), TNF-α, IFN-γ, and IL-1-β were increased. In summary, this parasite infects and replicates within adipocytes both in vitro and in vivo. Thus, we believe that adipocytes are an important target of parasite invasion. Chagas' disease is therefore an excellent model to study the contributions of adipose tissue in parasitic infections.
CONCLUDING REMARKS
This has been a targeted review, and clearly we have not performed an exhaustive review of every microorganism. For example, we have neither dealt with other parasites nor with the fungi nor with HIV/AIDS in detail. This review of the recent contributions from several laboratories, including our own, indicates that the adipose tissue and the adipocyte in particular contribute significantly to the systemic interaction with infectious agents. The adipocyte can be a direct target for a number of infectious agents and their products. These interactions have the potential to significantly impact the innate immune response. Adipose tissue plays a major role in inflammation and therefore is a major player in the pathogenesis of infectious diseases.
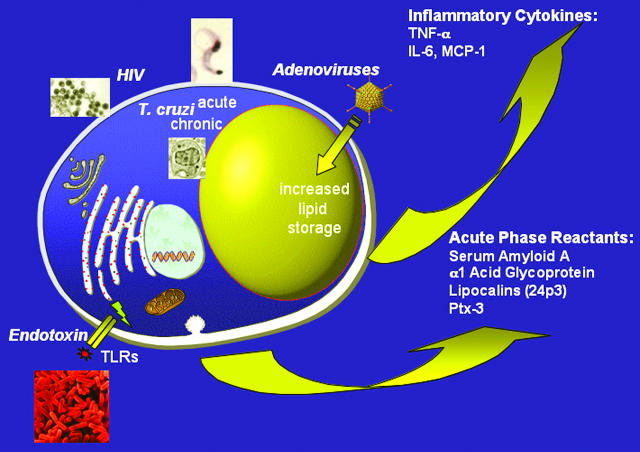
FIG. 1.
Schematic overview of the major infectious agents reported to target the adipocyte and the key adipokines release by adipocytes in response to local and systemic infection.
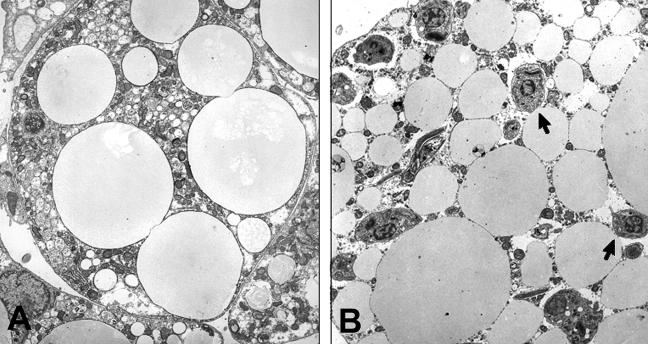
FIG. 2.
Representative electron micrograph of an uninfected 3T3-L1 adipocyte (A) and a cell 48 h after infection with trypomastigotes of T. cruzi. The arrows point to the amastigotes. (Reprinted from Combs et al. [25] with the permission of the publisher.)
TABLE 1.
Pathogens implicated in obesity
| Pathogena | Animal model | Possible mechanism(s) |
|---|---|---|
| Human adenovirus-36* | Chickens, mice, nonhuman primates | Upregulation of preadipocyte differentiation |
| Human adenovirus-37* | Chickens | Unknown |
| SMAM-1 adenovirus* | Chickens | Unknown |
| Borna disease virus* | Rats | Hypothalamic damage |
| Chlamydia pneumoniae* | No animal model, associated with wt gain in humans | Unknown |
| Scrapie agent | Mice | Hypothalamic-pituitary adrenal axis damage |
| Canine distemper virus | Mice | Hypothalamic damage, reduced hypothalamic leptin receptor expression |
| Rous-associated virus-7 | Chickens | Reduced thyroid hormone levels |
*, Human pathogens and/or associated with human obesity. Adapted from Dhurandhar et al. (30) with the permission of the publisher.
Acknowledgments
We acknowledge support from National Institutes of Health grants AI-068538 and AI-052739 (H.B.T.) and DK55758, CA112023, and DK071030 (P.E.S.). P.E.S. is a recipient of an Irma T. Hirschl Career Scientist Award. M.S.D. is supported by the IDSA ERF/NFID Colin L. Powell Minority Postdoctoral Fellowship in Tropical Disease Research sponsored by GlaxoSmithKline and by the NIH Training Grant in Mechanisms of Cardiovascular Diseases (T32 HL-07675). M.S.D. is a Neuroscience Fellow of the Dominick P. Purpura Department of Neuroscience and the Department of Psychiatry and Behavioral Sciences. M.E.T. was supported by a Mentor-Based Postdoctoral Fellowship Award from the American Diabetes Association (7-05-MI-09).
We thank Todd Schraw, Andrea Nawrocki, and Shankar Mukherjee for assistance with the graphic designs and Louis M. Weiss for helpful comments and discussions. In addition, we acknowledge the technical assistance of Vicki L. Braunstein, Dazhi Zhao, and of Frank Macaluso and Leslie Gunther of the Analytical Imaging Facility.
Editor: J. B. Kaper
Footnotes
Published ahead of print on 21 November 2006.
REFERENCES
- 1.Abir, F., and R. Bell. 2004. Assessment and management of the obese patient. Crit. Care Med. 32:S87-S91. [DOI] [PubMed] [Google Scholar]
- 2.Ahima, R. S., and J. S. Flier. 2000. Leptin. Annu. Rev. Physiol. 62:413-437. [DOI] [PubMed] [Google Scholar]
- 3.Ahima, R. S., D. Prabakaran, C. Mantzoros, D. Qu, B. Lowell, E. Maratos-Flier, and J. S. Flier. 1996. Role of leptin in the neuroendocrine response to fasting. Nature 382:250-252. [DOI] [PubMed] [Google Scholar]
- 4.Amole, B. O., M. Wittner, D. Hewlett, and H. B. Tanowitz. 1985. Trypanosoma brucei: infection in murine diabetes. Exp. Parasitol. 60:342-347. [DOI] [PubMed] [Google Scholar]
- 5.Annane, D., S. Sanquer, V. Sebille, A. Faye, D. Djuranovic, J. C. Raphael, P. Gajdos, and E. Bellissant. 2000. Compartmentalized inducible nitric-oxide synthase activity in septic shock. Lancet 355:1143-1148. [DOI] [PubMed] [Google Scholar]
- 6.Bacchetti, P., B. Gripshover, C. Grunfeld, S. Heymsfield, H. McCreath, D. Osmond, M. Saag, R. Scherzer, M. Shlipak, and P. Tien. 2005. Fat distribution in men with HIV infection. J. Acquir. Immune. Defic. Syndr. 40:121-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bado, A., S. Levasseur, S. Attoub, S. Kermorgant, J. P. Laigneau, M. N. Bortoluzzi, L. Moizo, T. Lehy, M. Guerre-Millo, Y. Le Marchand-Brustel, and M. J. Lewin. 1998. The stomach is a source of leptin. Nature 394:790-793. [DOI] [PubMed] [Google Scholar]
- 8.Barcan, L., C. Luna, L. Clara, A. Sinagra, A. Valledor, A. M. De Rissio, A. Gadano, M. M. Garcia, E. de Santibanes, and A. Riarte. 2005. Transmission of Trypanosoma cruzi infection via liver transplantation to a nonreactive recipient for Chagas' disease. Liver Transpl. 11:1112-1116. [DOI] [PubMed] [Google Scholar]
- 9.Bastard, J. P., M. Caron, H. Vidal, V. Jan, M. Auclair, C. Vigouroux, J. Luboinski, M. Laville, M. Maachi, P. M. Girard, W. Rozenbaum, P. Levan, and J. Capeau. 2002. Association between altered expression of adipogenic factor SREBP1 in lipoatrophic adipose tissue from HIV-1-infected patients and abnormal adipocyte differentiation and insulin resistance. Lancet 359:1026-1031. [DOI] [PubMed] [Google Scholar]
- 10.Bellmann, R., G. Kuchling, P. Dehghanyar, M. Zeitlinger, E. Minar, B. X. Mayer, M. Muller, and C. Joukhadar. 2004. Tissue pharmacokinetics of levofloxacin in human soft tissue infections. Br. J. Clin. Pharmacol. 57:563-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett, B. D., G. P. Solar, J. Q. Yuan, J. Mathias, G. R. Thomas, and W. Matthews. 1996. A role for leptin and its cognate receptor in hematopoiesis. Curr. Biol. 6:1170-1180. [DOI] [PubMed] [Google Scholar]
- 12.Bercault, N., T. Boulain, K. Kuteifan, M. Wolf, I. Runge, and J. C. Fleury. 2004. Obesity-related excess mortality rate in an adult intensive care unit: a risk-adjusted matched cohort study. Crit. Care Med. 32:998-1003. [DOI] [PubMed] [Google Scholar]
- 13.Berg, A. H., Y. Lin, M. P. Lisanti, and P. E. Scherer. 2004. Adipocyte differentiation induces dynamic changes in NF-κB expression and activity. Am. J. Physiol. Endocrinol. Metab. 287:E1178-E1188. [DOI] [PubMed] [Google Scholar]
- 14.Bleau, C., L. Lamontagne, and R. Savard. 2005. New Lactobacillus acidophilus isolates reduce the release of leptin by murine adipocytes leading to lower interferon-gamma production. Clin. Exp. Immunol. 140:427-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blumer, R. M., H. van Thien, A. F. Ruiter, G. J. Weverling, D. vinh Thuan, E. Endert, P. A. Kager, and H. P. Sauerwein. 2005. Adiponectin and glucose production in patients infected with Plasmodium falciparum. Metabolism 54:60-66. [DOI] [PubMed] [Google Scholar]
- 16.Bornstein, S. R., J. Licinio, R. Tauchnitz, L. Engelmann, A. B. Negrao, P. Gold, and G. P. Chrousos. 1998. Plasma leptin levels are increased in survivors of acute sepsis: associated loss of diurnal rhythm, in cortisol and leptin secretion. J. Clin. Endocrinol. Metab. 83:280-283. [DOI] [PubMed] [Google Scholar]
- 17.Bornstein, S. R., H. L. Preas, G. P. Chrousos, and A. F. Suffredini. 1998. Circulating leptin levels during acute experimental endotoxemia and anti-inflammatory therapy in humans. J. Infect. Dis. 178:887-890. [DOI] [PubMed] [Google Scholar]
- 18.Boulanger, B. R., D. P. Milzman, and A. Rodriguez. 1994. Obesity. Crit. Care Clin. 10:613-622. [PubMed] [Google Scholar]
- 19.Breidert, M., S. Miehlke, A. Glasow, Z. Orban, M. Stolte, G. Ehninger, E. Bayerdorffer, O. Nettesheim, U. Halm, A. Haidan, and S. R. Bornstein. 1999. Leptin and its receptor in normal human gastric mucosa and in Helicobacter pylori-associated gastritis. Scand. J. Gastroenterol. 34:954-961. [DOI] [PubMed] [Google Scholar]
- 20.Buerger, C., N. Plock, P. Dehghanyar, C. Joukhadar, and C. Kloft. 2006. Pharmacokinetics of unbound linezolid in plasma and tissue interstitium of critically ill patients after multiple dosing using microdialysis. Antimicrob. Agents Chemother. 50:2455-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Busso, N., A. So, V. Chobaz-Peclat, C. Morard, E. Martinez-Soria, D. Talabot-Ayer, and C. Gabay. 2002. Leptin signaling deficiency impairs humoral and cellular immune responses and attenuates experimental arthritis. J. Immunol. 168:875-882. [DOI] [PubMed] [Google Scholar]
- 22.Carlson, G. L., M. Saeed, R. A. Little, and M. H. Irving. 1999. Serum leptin concentrations and their relation to metabolic abnormalities in human sepsis. Am. J. Physiol. 276:E658-E662. [DOI] [PubMed] [Google Scholar]
- 23.Carlson, M. G., W. L. Snead, A. M. Oeser, and M. G. Butler. 1999. Plasma leptin concentrations in lean and obese human subjects and Prader-Willi syndrome: comparison of RIA and ELISA methods. J. Lab. Clin. Med. 133:75-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carter, J. K., and R. E. Smith. 1984. Specificity of avian leukosis virus-induced hyperlipidemia. J. Virol. 50:301-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Combs, T. P., Nagajyothi, S. Mukherjee, C. J. de Almeida, L. A. Jelicks, W. Schubert, Y. Lin, D. S. Jayabalan, D. Zhao, V. L. Braunstein, S. Landskroner-Eiger, A. Cordero, S. M. Factor, L. M. Weiss, M. P. Lisanti, H. B. Tanowitz, and P. E. Scherer. 2005. The adipocyte as an important target cell for Trypanosoma cruzi infection. J. Biol. Chem. 280:24085-24094. [DOI] [PubMed] [Google Scholar]
- 26.Cook, K. S., H. Y. Min, D. Johnson, R. J. Chaplinsky, J. S. Flier, C. R. Hunt, and B. M. Spiegelman. 1987. Adipsin: a circulating serine protease homolog secreted by adipose tissue and sciatic nerve. Science 237:402-405. [DOI] [PubMed] [Google Scholar]
- 27.Dandona, P., A. Aljada, S. Dhindsa, and R. Garg. 2003. Insulin as an anti-inflammatory and antiatherosclerotic hormone. Clin. Cornerstone 2003(Suppl. 4):S13-S20. [DOI] [PubMed] [Google Scholar]
- 28.Dhurandhar, N. V. 2004. Contribution of pathogens in human obesity. Drug News Perspect. 17:307-313. [DOI] [PubMed] [Google Scholar]
- 29.Dhurandhar, N. V. 2001. Infectobesity: obesity of infectious origin. J. Nutr. 131:2794S-2797S. [DOI] [PubMed] [Google Scholar]
- 30.Dhurandhar, N. V., R. L. Atkinson, and A. Ahmed. 2004. Obesity of infectious origin: a review. Growth Genetics Hormones 20:33-39. [Google Scholar]
- 31.Dhurandhar, N. V., B. A. Israel, J. M. Kolesar, G. F. Mayhew, M. E. Cook, and R. L. Atkinson. 2000. Increased adiposity in animals due to a human virus. Int. J. Obes. Relat. Metab. Disord. 24:989-996. [DOI] [PubMed] [Google Scholar]
- 32.Dhurandhar, N. V., P. R. Kulkarni, S. M. Ajinkya, A. A. Sherikar, and R. L. Atkinson. 1997. Association of adenovirus infection with human obesity. Obes. Res. 5:464-469. [DOI] [PubMed] [Google Scholar]
- 33.Dhurandhar, N. V., L. D. Whigham, D. H. Abbott, N. J. Schultz-Darken, B. A. Israel, S. M. Bradley, J. W. Kemnitz, D. B. Allison, and R. L. Atkinson. 2002. Human adenovirus Ad-36 promotes weight gain in male rhesus and marmoset monkeys. J. Nutr. 132:3155-3160. [DOI] [PubMed] [Google Scholar]
- 34.Domingo, P., X. Matias-Guiu, R. M. Pujol, E. Francia, E. Lagarda, M. A. Sambeat, and G. Vazquez. 1999. Subcutaneous adipocyte apoptosis in HIV-1 protease inhibitor-associated lipodystrophy. AIDS 13:2261-2267. [DOI] [PubMed] [Google Scholar]
- 35.dos Santos, V. M., S. F. da Cunha, P. Teixeira Vde, J. P. Monteiro, J. A. dos Santos, T. A. dos Santos, L. A. dos Santos, and D. F. da Cunha. 1999. Frequency of diabetes mellitus and hyperglycemia in chagasic and non-chagasic women. Rev. Soc. Bras. Med. Trop. 32:489-496. [DOI] [PubMed] [Google Scholar]
- 36.Fajas, L., K. Schoonjans, L. Gelman, J. B. Kim, J. Najib, G. Martin, J. C. Fruchart, M. Briggs, B. M. Spiegelman, and J. Auwerx. 1999. Regulation of peroxisome proliferator-activated receptor gamma expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: implications for adipocyte differentiation and metabolism. Mol. Cell. Biol. 19:5495-5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fasshauer, M., J. Klein, S. Neumann, M. Eszlinger, and R. Paschke. 2002. Hormonal regulation of adiponectin gene expression in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 290:1084-1089. [DOI] [PubMed] [Google Scholar]
- 38.Feingold, K. R., and C. Grunfeld. 1992. Role of cytokines in inducing hyperlipidemia. Diabetes 41(Suppl. 2):97-101. [DOI] [PubMed] [Google Scholar]
- 39.Fukuhara, A., M. Matsuda, M. Nishizawa, K. Segawa, M. Tanaka, K. Kishimoto, Y. Matsuki, M. Murakami, T. Ichisaka, H. Murakami, E. Watanabe, T. Takagi, M. Akiyoshi, T. Ohtsubo, S. Kihara, S. Yamashita, M. Makishima, T. Funahashi, S. Yamanaka, R. Hiramatsu, Y. Matsuzawa, and I. Shimomura. 2005. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science 307:426-430. [DOI] [PubMed] [Google Scholar]
- 40.Gainsford, T., T. A. Willson, D. Metcalf, E. Handman, C. McFarlane, A. Ng, N. A. Nicola, W. S. Alexander, and D. J. Hilton. 1996. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc. Natl. Acad. Sci. USA 93:14564-14568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gosztonyi, G., and H. Ludwig. 1995. Borna disease-neuropathology and pathogenesis. Curr. Top. Microbiol. Immunol. 190:39-73. [PubMed] [Google Scholar]
- 42.Greenway, F. 2006. Virus-induced obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290:R188-E189. [DOI] [PubMed] [Google Scholar]
- 43.Grinspoon, S., and A. Carr. 2005. Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N. Engl. J. Med. 352:48-62. [DOI] [PubMed] [Google Scholar]
- 44.Guariento, M. E., M. J. Saad, E. O. Muscelli, and J. A. Gontijo. 1993. Heterogenous insulin response to an oral glucose load by patients with the indeterminate clinical form of Chagas' disease. Braz. J. Med. Biol. Res. 26:491-495. [PubMed] [Google Scholar]
- 45.Hadigan, C., C. Corcoran, S. Piecuch, W. Rodriguez, and S. Grinspoon. 2000. Hyperandrogenemia in human immunodeficiency virus-infected women with the lipodystrophy syndrome. J. Clin. Endocrinol. Metab. 85:3544-3550. [DOI] [PubMed] [Google Scholar]
- 46.Hazan, U., I. A. Romero, R. Cancello, S. Valente, V. Perrin, V. Mariot, J. Dumonceaux, C. C. Gerhardt, A. D. Strosberg, P. O. Couraud, and F. Pietri-Rouxel. 2002. Human adipose cells express CD4, CXCR4, and CCR5 [corrected] receptors: a new target cell type for the immunodeficiency virus-1? FASEB J. 16:1254-1256. [DOI] [PubMed] [Google Scholar]
- 47.Hill, R. A., S. Margetic, G. G. Pegg, and C. Gazzola. 1998. Leptin: its pharmacokinetics and tissue distribution. Int. J. Obes. Relat. Metab. Disord. 22:765-770. [DOI] [PubMed] [Google Scholar]
- 48.Hoffmann, G., M. Czechowski, M. Schloesser, and W. Schobersberger. 2002. Procalcitonin amplifies inducible nitric oxide synthase gene expression and nitric oxide production in vascular smooth muscle cells. Crit. Care Med. 30:2091-2095. [DOI] [PubMed] [Google Scholar]
- 49.Holscher, C., M. Mohrs, W. J. Dai, G. Kohler, B. Ryffel, G. A. Schaub, H. Mossmann, and F. Brombacher. 2000. Tumor necrosis factor alpha-mediated toxic shock in Trypanosoma cruzi-infected interleukin 10-deficient mice. Infect. Immun. 68:4075-4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hotamisligil, G. S., N. S. Shargill, and B. M. Spiegelman. 1993. Adipose tissue expression of tumor necrosis factor alpha: direct role in obesity-linked insulin resistance. Science 259:87-90. [DOI] [PubMed] [Google Scholar]
- 51.Igarashi, M., K. Yamatani, N. Fukase, M. Daimon, H. Ohnuma, H. Takahashi, H. Manaka, M. Tominaga, and H. Sasaki. 1992. Sepsis inhibits insulin-stimulated glucose transport in isolated rat adipocytes. Diabetes Res. Clin. Pract. 15:213-218. [DOI] [PubMed] [Google Scholar]
- 52.Jan, V., P. Cervera, M. Maachi, M. Baudrimont, M. Kim, H. Vidal, P. M. Girard, P. Levan, W. Rozenbaum, A. Lombes, J. Capeau, and J. P. Bastard. 2004. Altered fat differentiation and adipocytokine expression are inter-related and linked to morphological changes and insulin resistance in HIV-1-infected lipodystrophic patients. Antivir. Ther. 9:555-564. [PubMed] [Google Scholar]
- 53.Johnson, J. A., J. B. Albu, E. S. Engelson, S. K. Fried, Y. Inada, G. Ionescu, and D. P. Kotler. 2004. Increased systemic and adipose tissue cytokines in patients with HIV-associated lipodystrophy. Am. J. Physiol. Endocrinol. Metab. 286:E261-E271. [DOI] [PubMed] [Google Scholar]
- 54.Joukhadar, C., M. Frossard, B. X. Mayer, M. Brunner, N. Klein, P. Siostrzonek, H. G. Eichler, and M. Muller. 2001. Impaired target site penetration of beta-lactams may account for therapeutic failure in patients with septic shock. Crit. Care Med. 29:385-391. [DOI] [PubMed] [Google Scholar]
- 55.Kamon, J., T. Yamauchi, S. Muto, S. Takekawa, Y. Ito, Y. Hada, W. Ogawa, A. Itai, M. Kasuga, K. Tobe, and T. Kadowaki. 2004. A novel IKK β inhibitor stimulates adiponectin levels and ameliorates obesity-linked insulin resistance. Biochem. Biophys. Res. Commun. 323:242-248. [DOI] [PubMed] [Google Scholar]
- 56.Keller, P., K. Moller, K. S. Krabbe, and B. K. Pedersen. 2003. Circulating adiponectin levels during human endotoxaemia. Clin. Exp. Immunol. 134:107-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kellerer, M., M. Koch, E. Metzinger, J. Mushack, E. Capp, and H. U. Haring. 1997. Leptin activates PI-3 kinase in C2C12 myotubes via Janus kinase-2 (JAK-2) and insulin receptor substrate-2 (IRS-2) dependent pathways. Diabetologia 40:1358-1362. [DOI] [PubMed] [Google Scholar]
- 58.Khovidhunkit, W., M. S. Kim, R. A. Memon, J. K. Shigenaga, A. H. Moser, K. R. Feingold, and C. Grunfeld. 2004. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J. Lipid Res. 45:1169-1196. [DOI] [PubMed] [Google Scholar]
- 59.Kida, Y., A. Esposito-Del Puente, C. Bogardus, and D. M. Mott. 1990. Insulin resistance is associated with reduced fasting and insulin-stimulated glycogen synthase phosphatase activity in human skeletal muscle. J. Clin. Investig. 85:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim, Y. S., R. I. Carp, S. M. Callahan, and H. M. Wisniewski. 1988. Adrenal involvement in scrapie-induced obesity. Proc. Soc. Exp. Biol. Med. 189:21-27. [DOI] [PubMed] [Google Scholar]
- 61.Kosmiski, L., D. Kuritzkes, K. Lichtenstein, and R. Eckel. 2003. Adipocyte-derived hormone levels in HIV lipodystrophy. Antivir. Ther. 8:9-15. [PubMed] [Google Scholar]
- 62.Lagathu, C., J. P. Bastard, M. Auclair, M. Maachi, M. Kornprobst, J. Capeau, and M. Caron. 2004. Antiretroviral drugs with adverse effects on adipocyte lipid metabolism and survival alter the expression and secretion of proinflammatory cytokines and adiponectin in vitro. Antivir. Ther. 9:911-920. [PubMed] [Google Scholar]
- 63.Lages-Silva, E., L. E. Ramirez, M. L. Silva-Vergara, and E. Chiari. 2002. Chagasic meningoencephalitis in a patient with acquired immunodeficiency syndrome: diagnosis, follow-up, and genetic characterization of Trypanosoma cruzi. Clin. Infect. Dis. 34:118-123. [DOI] [PubMed] [Google Scholar]
- 64.Lanza-Jacoby, S., S. C. Lansey, M. P. Cleary, and F. E. Rosato. 1982. Alterations in lipogenic enzymes and lipoprotein lipase activity during gram-negative sepsis in the rat. Arch. Surg. 117:144-147. [DOI] [PubMed] [Google Scholar]
- 65.Lanza-Jacoby, S., and A. Tabares. 1990. Triglyceride kinetics, tissue lipoprotein lipase, and liver lipogenesis in septic rats. Am. J. Physiol. 258:E678-E685. [DOI] [PubMed] [Google Scholar]
- 66.Leiby, D. A., R. M. Herron, Jr., E. J. Read, B. A. Lenes, and R. J. Stumpf. 2002. Trypanosoma cruzi in Los Angeles and Miami blood donors: impact of evolving donor demographics on seroprevalence and implications for transfusion transmission. Transfusion 42:549-555. [DOI] [PubMed] [Google Scholar]
- 67.Lilienfeld, D. E., D. Vlahov, J. H. Tenney, and J. S. McLaughlin. 1988. Obesity and diabetes as risk factors for postoperative wound infections after cardiac surgery. Am. J. Infect. Control. 16:3-6. [DOI] [PubMed] [Google Scholar]
- 68.Lin, Y., A. H. Berg, P. Iyengar, T. K. Lam, A. Giacca, T. P. Combs, M. W. Rajala, X. Du, B. Rollman, W. Li, M. Hawkins, N. Barzilai, C. J. Rhodes, I. G. Fantus, M. Brownlee, and P. E. Scherer. 2005. The hyperglycemia-induced inflammatory response in adipocytes: the role of reactive oxygen species. J. Biol. Chem. 280:4617-4626. [DOI] [PubMed] [Google Scholar]
- 69.Lin, Y., H. Lee, A. H. Berg, M. P. Lisanti, L. Shapiro, and P. E. Scherer. 2000. LPS activated TLR-4 receptor induces synthesis of the closely related receptor TLR-2 in adipocytes. J. Biol. Chem. 275:24255-24263. [DOI] [PubMed] [Google Scholar]
- 70.Lin, Y., M. W. Rajala, J. P. Berger, D. E. Moller, N. Barzilai, and P. E. Scherer. 2001. Hyperglycemia-induced production of acute phase reactants in adipose tissue. J. Biol. Chem. 276:42077-42083. [DOI] [PubMed] [Google Scholar]
- 71.Linscheid, P., D. Seboek, E. S. Nylen, I. Langer, M. Schlatter, K. L. Becker, U. Keller, and B. Muller. 2003. In vitro and in vivo calcitonin I gene expression in parenchymal cells: a novel product of human adipose tissue. Endocrinology 144:5578-5584. [DOI] [PubMed] [Google Scholar]
- 72.Linscheid, P., D. Seboek, D. J. Schaer, H. Zulewski, U. Keller, and B. Muller. 2004. Expression and secretion of procalcitonin and calcitonin gene-related peptide by adherent monocytes and by macrophage-activated adipocytes. Crit. Care Med. 32:1715-1721. [DOI] [PubMed] [Google Scholar]
- 73.Linscheid, P., D. Seboek, H. Zulewski, U. Keller, and B. Muller. 2005. Autocrine/paracrine role of inflammation-mediated calcitonin gene-related peptide and adrenomedullin expression in human adipose tissue. Endocrinology 146:2699-2708. [DOI] [PubMed] [Google Scholar]
- 74.Loffreda, S., S. Q. Yang, H. Z. Lin, C. L. Karp, M. L. Brengman, D. J. Wang, A. S. Klein, G. B. Bulkley, C. Bao, P. W. Noble, M. D. Lane, and A. M. Diehl. 1998. Leptin regulates proinflammatory immune responses. FASEB J. 12:57-65. [PubMed] [Google Scholar]
- 75.Lord, G. M., G. Matarese, J. K. Howard, R. J. Baker, S. R. Bloom, and R. I. Lechler. 1998. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394:897-901. [DOI] [PubMed] [Google Scholar]
- 76.Lyons, M. J., I. M. Faust, R. B. Hemmes, D. R. Buskirk, J. Hirsch, and J. B. Zabriskie. 1982. A virally induced obesity syndrome in mice. Science 216:82-85. [DOI] [PubMed] [Google Scholar]
- 77.Maeda, N., I. Shimomura, K. Kishida, H. Nishizawa, M. Matsuda, H. Nagaretani, N. Furuyama, H. Kondo, M. Takahashi, Y. Arita, R. Komuro, N. Ouchi, S. Kihara, Y. Tochino, K. Okutomi, M. Horie, S. Takeda, T. Aoyama, T. Funahashi, and Y. Matsuzawa. 2002. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 8:731-737. [DOI] [PubMed] [Google Scholar]
- 78.Maffei, M., H. Fei, G. H. Lee, C. Dani, P. Leroy, Y. Zhang, R. Proenca, R. Negrel, G. Ailhaud, and J. M. Friedman. 1995. Increased expression in adipocytes of ob RNA in mice with lesions of the hypothalamus and with mutations at the db locus. Proc. Natl. Acad. Sci. USA 92:6957-6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mancuso, P., A. Gottschalk, S. M. Phare, M. Peters-Golden, N. W. Lukacs, and G. B. Huffnagle. 2002. Leptin-deficient mice exhibit impaired host defense in gram-negative pneumonia. J. Immunol. 168:4018-4024. [DOI] [PubMed] [Google Scholar]
- 80.Marik, P., and J. Varon. 1998. The obese patient in the ICU. Chest 113:492-498. [DOI] [PubMed] [Google Scholar]
- 81.Martin-Romero, C., J. Santos-Alvarez, R. Goberna, and V. Sanchez- Margalet. 2000. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell. Immunol. 199:15-24. [DOI] [PubMed] [Google Scholar]
- 82.Masuzaki, H., Y. Ogawa, N. Sagawa, K. Hosoda, T. Matsumoto, H. Mise, H. Nishimura, Y. Yoshimasa, I. Tanaka, T. Mori, and K. Nakao. 1997. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat. Med. 3:1029-1033. [DOI] [PubMed] [Google Scholar]
- 83.Maurin, T., C. Saillan-Barreau, B. Cousin, L. Casteilla, A. Doglio, and L. Penicaud. 2005. Tumor necrosis factor-alpha stimulates HIV-1 production in primary culture of human adipocytes. Exp. Cell Res. 304:544-551. [DOI] [PubMed] [Google Scholar]
- 84.Memon, R. A., K. R. Feingold, A. H. Moser, J. Fuller, and C. Grunfeld. 1998. Regulation of fatty acid transport protein and fatty acid translocase mRNA levels by endotoxin and cytokines. Am. J. Physiol. 274:E210-E217. [DOI] [PubMed] [Google Scholar]
- 85.Mohamed-Ali, V., S. Goodrick, A. Rawesh, D. R. Katz, J. M. Miles, J. S. Yudkin, S. Klein, and S. W. Coppack. 1997. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J. Clin. Endocrinol. Metab. 82:4196-4200. [DOI] [PubMed] [Google Scholar]
- 86.Moore, S. I., G. B. Huffnagle, G. H. Chen, E. S. White, and P. Mancuso. 2003. Leptin modulates neutrophil phagocytosis of Klebsiella pneumoniae. Infect. Immun. 71:4182-4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Munier, S., A. Borjabad, M. Lemaire, V. Mariot, and U. Hazan. 2003. In vitro infection of human primary adipose cells with HIV-1: a reassessment. AIDS 17:2537-2539. [DOI] [PubMed] [Google Scholar]
- 88.Mynarcik, D., L. X. Wei, E. Komaroff, R. Ferris, M. McNurlan, and M. Gelato. 2005. Chronic loss of subcutaneous adipose tissue in HIV-associated lipodystrophy may not be associated with accelerated apoptosis. J. Acquir. Immune Defic. Syndr. 38:367-371. [PubMed] [Google Scholar]
- 89.Mynarcik, D. C., T. Combs, M. A. McNurlan, P. E. Scherer, E. Komaroff, and M. C. Gelato. 2002. Adiponectin and leptin levels in HIV-infected subjects with insulin resistance and body fat redistribution. J. Acquir. Immune. Defic. Syndr. 31:514-520. [DOI] [PubMed] [Google Scholar]
- 90.Mynarcik, D. C., M. A. McNurlan, R. T. Steigbigel, J. Fuhrer, and M. C. Gelato. 2000. Association of severe insulin resistance with both loss of limb fat and elevated serum tumor necrosis factor receptor levels in HIV lipodystrophy. J. Acquir. Immune. Defic. Syndr. 25:312-321. [DOI] [PubMed] [Google Scholar]
- 91.Nedrebo, T., T. V. Karlsen, G. S. Salvesen, and R. K. Reed. 2004. A novel function of insulin in rat dermis. J. Physiol. 559:583-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nishikawa, T., D. Edelstein, X. L. Du, S. Yamagishi, T. Matsumura, Y. Kaneda, M. A. Yorek, D. Beebe, P. J. Oates, H. P. Hammes, I. Giardino, and M. Brownlee. 2000. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 404:787-790. [DOI] [PubMed] [Google Scholar]
- 93.Nolan, D., E. Hammond, A. Martin, L. Taylor, S. Herrmann, E. McKinnon, C. Metcalf, B. Latham, and S. Mallal. 2003. Mitochondrial DNA depletion and morphologic changes in adipocytes associated with nucleoside reverse transcriptase inhibitor therapy. AIDS 17:1329-1338. [DOI] [PubMed] [Google Scholar]
- 94.Nylen, E. S., K. T. Whang, R. H. Snider, Jr., P. M. Steinwald, J. C. White, and K. L. Becker. 1998. Mortality is increased by procalcitonin and decreased by an antiserum reactive to procalcitonin in experimental sepsis. Crit. Care Med. 26:1001-1006. [DOI] [PubMed] [Google Scholar]
- 95.Okamoto, Y., Y. Arita, M. Nishida, M. Muraguchi, N. Ouchi, M. Takahashi, T. Igura, Y. Inui, S. Kihara, T. Nakamura, S. Yamashita, J. Miyagawa, T. Funahashi, and Y. Matsuzawa. 2000. An adipocyte-derived plasma protein, adiponectin, adheres to injured vascular walls. Horm. Metab. Res. 32:47-50. [DOI] [PubMed] [Google Scholar]
- 96.Oliveira, L. C., Y. Juliano, N. F. Novo, and M. M. Neves. 1993. Blood glucose and insulin response to intravenous glucose by patients with chronic Chagas' disease and alcoholism. Braz. J. Med. Biol. Res. 26:1187-1190. [PubMed] [Google Scholar]
- 97.Ouchi, N., S. Kihara, Y. Arita, K. Maeda, H. Kuriyama, Y. Okamoto, K. Hotta, M. Nishida, M. Takahashi, T. Nakamura, S. Yamashita, T. Funahashi, and Y. Matsuzawa. 1999. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation 100:2473-2476. [DOI] [PubMed] [Google Scholar]
- 98.Ouchi, N., S. Kihara, Y. Arita, Y. Okamoto, K. Maeda, H. Kuriyama, K. Hotta, M. Nishida, M. Takahashi, M. Muraguchi, Y. Ohmoto, T. Nakamura, S. Yamashita, T. Funahashi, and Y. Matsuzawa. 2000. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-κB signaling through a cAMP-dependent pathway. Circulation 102:1296-1301. [DOI] [PubMed] [Google Scholar]
- 99.Ozata, M., I. C. Ozdemir, and J. Licinio. 1999. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J. Clin. Endocrinol. Metab. 84:3686-3695. [DOI] [PubMed] [Google Scholar]
- 100.Pajvani, U. B., M. E. Trujillo, T. P. Combs, P. Iyengar, L. Jelicks, K. A. Roth, R. N. Kitsis, and P. E. Scherer. 2005. Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat. Med. 11:797-803. [DOI] [PubMed] [Google Scholar]
- 101.Reichlin, S. 1983. Somatostatin. N. Engl. J. Med. 309:1495-1501. [DOI] [PubMed] [Google Scholar]
- 102.Rocha, A., A. C. de Meneses, A. M. da Silva, M. S. Ferreira, S. A. Nishioka, M. K. Burgarelli, E. Almeida, G. Turcato Junior, K. Metze, and E. R. Lopes. 1994. Pathology of patients with Chagas' disease and acquired immunodeficiency syndrome. Am. J. Trop. Med. Hyg. 50:261-268. [DOI] [PubMed] [Google Scholar]
- 103.Rosato, E. F., P. Vemulapalli, C. H. Lang, and S. Lanza-Jacoby. 1997. Insulin stimulates lipoprotein lipase activity and synthesis in adipocytes from septic rats. J. Surg. Res. 73:73-79. [DOI] [PubMed] [Google Scholar]
- 104.Rosen, E. D., C. J. Walkey, P. Puigserver, and B. M. Spiegelman. 2000. Transcriptional regulation of adipogenesis. Genes Dev. 14:1293-1307. [PubMed] [Google Scholar]
- 105.Ruan, H., and H. F. Lodish. 2003. Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-alpha. Cytokine Growth Factor Rev. 14:447-455. [DOI] [PubMed] [Google Scholar]
- 106.Sanchez-Margalet, V., and C. Martin-Romero. 2001. Human leptin signaling in human peripheral blood mononuclear cells: activation of the JAK-STAT pathway. Cell. Immunol. 211:30-36. [DOI] [PubMed] [Google Scholar]
- 107.Santos-Alvarez, J., R. Goberna, and V. Sanchez-Margalet. 1999. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell. Immunol. 194:6-11. [DOI] [PubMed] [Google Scholar]
- 108.Sarraf, P., R. C. Frederich, E. M. Turner, G. Ma, N. T. Jaskowiak, D. J. Rivet III, J. S. Flier, B. B. Lowell, D. L. Fraker, and H. R. Alexander. 1997. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J. Exp. Med. 185:171-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sartori, A. M., H. H. Caiaffa-Filho, R. C. Bezerra, S. G. C. do, M. H. Lopes, and M. A. Shikanai-Yasuda. 2002. Exacerbation of HIV viral load simultaneous with asymptomatic reactivation of chronic Chagas' disease. Am. J. Trop. Med. Hyg. 67:521-523. [DOI] [PubMed] [Google Scholar]
- 110.Sartori, A. M., M. H. Lopes, L. A. Benvenuti, B. Caramelli, A. di Pietro, E. V. Nunes, L. P. Ramirez, and M. A. Shikanai-Yasuda. 1998. Reactivation of Chagas' disease in a human immunodeficiency virus-infected patient leading to severe heart disease with a late positive direct microscopic examination of the blood. Am. J. Trop. Med. Hyg. 59:784-786. [DOI] [PubMed] [Google Scholar]
- 111.Sartori, A. M., J. E. Neto, E. V. Nunes, L. M. Braz, H. H. Caiaffa-Filho, C. Oliveira Oda, Jr., V. A. Neto, and M. A. Shikanai-Yasuda. 2002. Trypanosoma cruzi parasitemia in chronic Chagas disease: comparison between human immunodeficiency virus (HIV)-positive and HIV-negative patients. J. Infect. Dis. 186:872-875. [DOI] [PubMed] [Google Scholar]
- 112.Sauermann, R., M. Zeitlinger, B. M. Erovic, C. Marsik, A. Georgopoulos, M. Muller, M. Brunner, and C. Joukhadar. 2003. Pharmacodynamics of piperacillin in severely ill patients evaluated by using a PK/PD model. Int. J. Antimicrob. Agents 22:574-578. [DOI] [PubMed] [Google Scholar]
- 113.Scarborough, D. E. 1990. Cytokine modulation of pituitary hormone secretion. Ann. N. Y. Acad. Sci. 594:169-187. [DOI] [PubMed] [Google Scholar]
- 114.Scarborough, D. E., S. L. Lee, C. A. Dinarello, and S. Reichlin. 1989. Interleukin-1 beta stimulates somatostatin biosynthesis in primary cultures of fetal rat brain. Endocrinology 124:549-551. [DOI] [PubMed] [Google Scholar]
- 115.Scherer, P. E., S. Williams, M. Fogliano, G. Baldini, and H. F. Lodish. 1995. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 270:26746-26749. [DOI] [PubMed] [Google Scholar]
- 116.Seboek, D., P. Linscheid, H. Zulewski, I. Langer, M. Christ-Crain, U. Keller, and B. Muller. 2004. Somatostatin is expressed and secreted by human adipose tissue upon infection and inflammation. J. Clin. Endocrinol. Metab. 89:4833-4839. [DOI] [PubMed] [Google Scholar]
- 117.Shibata, R., K. Sato, D. R. Pimentel, Y. Takemura, S. Kihara, K. Ohashi, T. Funahashi, N. Ouchi, and K. Walsh. 2005. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat. Med. 11:1096-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shimomura, I., R. E. Hammer, J. A. Richardson, S. Ikemoto, Y. Bashmakov, J. L. Goldstein, and M. S. Brown. 1998. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev. 12:3182-3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shulman, G. I. 2000. Cellular mechanisms of insulin resistance. J. Clin. Investig. 106:171-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Steinwald, P. M., K. T. Whang, K. L. Becker, R. H. Snider, E. S. Nylen, and J. C. White. 1999. Elevated calcitonin precursor levels are related to mortality in an animal model of sepsis. Crit. Care 3:11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Steppan, C. M., S. T. Bailey, S. Bhat, E. J. Brown, R. R. Banerjee, C. M. Wright, H. R. Patel, R. S. Ahima, and M. A. Lazar. 2001. The hormone resistin links obesity to diabetes. Nature 409:307-312. [DOI] [PubMed] [Google Scholar]
- 122.Tanabe, K., S. Okuya, Y. Tanizawa, A. Matsutani, and Y. Oka. 1997. Leptin induces proliferation of pancreatic beta cell line MIN6 through activation of mitogen-activated protein kinase. Biochem. Biophys. Res. Commun. 241:765-768. [DOI] [PubMed] [Google Scholar]
- 123.Tanowitz, H. B., B. Amole, D. Hewlett, and M. Wittner. 1988. Trypanosoma cruzi infection in diabetic mice. Trans R. Soc. Trop. Med. Hyg. 82:90-93. [PubMed] [Google Scholar]
- 124.Tartaglia, L. A., M. Dembski, X. Weng, N. Deng, J. Culpepper, R. Devos, G. J. Richards, L. A. Campfield, F. T. Clark, J. Deeds, et al. 1995. Identification and expression cloning of a leptin receptor, OB-R. Cell 83:1263-1271. [DOI] [PubMed] [Google Scholar]
- 125.Trujillo, M. E., M. J. Lee, S. Sullivan, J. Feng, S. H. Schneider, A. S. Greenberg, and S. K. Fried. 2006. Tumor necrosis factor alpha and glucocorticoid synergistically increase leptin production in human adipose tissue: role for p38 mitogen-activated protein kinase. J. Clin. Endocrinol. Metab. 91:1484-1490. [DOI] [PubMed] [Google Scholar]
- 126.Tsuchihashi, H., H. Yamamoto, K. Maeda, S. Ugi, T. Mori, T. Shimizu, Y. Endo, K. Hanasawa, and T. Tani. 2006. Circulating concentrations of adiponectin, an endogenous lipopolysaccharide neutralizing protein, decrease in rats with polymicrobial sepsis. J. Surg. Res. 134:348-353. [DOI] [PubMed] [Google Scholar]
- 127.Uysal, K. T., S. M. Wiesbrock, M. W. Marino, and G. S. Hotamisligil. 1997. Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature 389:610-614. [DOI] [PubMed] [Google Scholar]
- 128.Vaidian, A. K., L. M. Weiss, and H. B. Tanowitz. 2004. Chagas' disease and AIDS. Kinetoplastid Biol. Dis. 3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.van den Berghe, G., P. Wouters, F. Weekers, C. Verwaest, F. Bruyninckx, M. Schetz, D. Vlasselaers, P. Ferdinande, P. Lauwers, and R. Bouillon. 2001. Intensive insulin therapy in the critically ill patients. N. Engl. J. Med. 345:1359-1367. [DOI] [PubMed] [Google Scholar]
- 130.Vorbrodt, A. W., D. H. Dobrogowska, M. Tarnawski, H. C. Meeker, and R. I. Carp. 2001. Quantitative immunogold study of glucose transporter (GLUT-1) in five brain regions of scrapie-infected mice showing obesity and reduced glucose tolerance. Acta Neuropathol. 102:278-284. [DOI] [PubMed] [Google Scholar]
- 131.Wannemacher, R. W., Jr., J. G. Pace, R. A. Beall, R. E. Dinterman, V. J. Petrella, and H. A. Neufeld. 1979. Role of the liver in regulation of ketone body production during sepsis. J. Clin. Investig. 64:1565-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Weisberg, S. P., D. McCann, M. Desai, M. Rosenbaum, R. L. Leibel, and A. W. Ferrante, Jr. 2003. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 112:1796-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Whang, K. T., P. M. Steinwald, J. C. White, E. S. Nylen, R. H. Snider, G. L. Simon, R. L. Goldberg, and K. L. Becker. 1998. Serum calcitonin precursors in sepsis and systemic inflammation. J. Clin. Endocrinol. Metab. 83:3296-3301. [DOI] [PubMed] [Google Scholar]
- 134.Whigham, L. D., B. A. Israel, and R. L. Atkinson. 2006. Adipogenic potential of multiple human adenoviruses in vivo and in vitro in animals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290:R190-R194. [DOI] [PubMed] [Google Scholar]
- 135.Wieland, C. W., S. Florquin, E. D. Chan, J. C. Leemans, S. Weijer, A. Verbon, G. Fantuzzi, and T. van der Poll. 2005. Pulmonary Mycobacterium tuberculosis infection in leptin-deficient ob/ob mice. Int. Immunol. 17:1399-1408. [DOI] [PubMed] [Google Scholar]
- 136.Xu, H., G. T. Barnes, Q. Yang, G. Tan, D. Yang, C. J. Chou, J. Sole, A. Nichols, J. S. Ross, L. A. Tartaglia, and H. Chen. 2003. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 112:1821-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yamauchi, T., J. Kamon, Y. Minokoshi, Y. Ito, H. Waki, S. Uchida, S. Yamashita, M. Noda, S. Kita, K. Ueki, K. Eto, Y. Akanuma, P. Froguel, F. Foufelle, P. Ferre, D. Carling, S. Kimura, R. Nagai, B. B. Kahn, and T. Kadowaki. 2002. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 8:1288-1295. [DOI] [PubMed] [Google Scholar]
- 138.Yang, Q., T. E. Graham, N. Mody, F. Preitner, O. D. Peroni, J. M. Zabolotny, K. Kotani, L. Quadro, and B. B. Kahn. 2005. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436:356-362. [DOI] [PubMed] [Google Scholar]
- 139.Yang, R. Z., M. J. Lee, H. Hu, J. Pray, H. B. Wu, B. C. Hansen, A. R. Shuldiner, S. K. Fried, J. C. McLenithan, and D. W. Gong. 2006. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am. J. Physiol. Endocrinol. Metab. 290:E1253-E1261. [DOI] [PubMed] [Google Scholar]
- 140.Zeitlinger, M. A., B. M. Erovic, R. Sauermann, A. Georgopoulos, M. Muller, and C. Joukhadar. 2005. Plasma concentrations might lead to overestimation of target site activity of piperacillin in patients with sepsis. J. Antimicrob. Chemother. 56:703-708. [DOI] [PubMed] [Google Scholar]
- 141.Zhang, Y., R. Proenca, M. Maffei, M. Barone, L. Leopold, and J. M. Friedman. 1994. Positional cloning of the mouse obese gene and its human homologue. Nature 372:425-432. [DOI] [PubMed] [Google Scholar]