Abstract
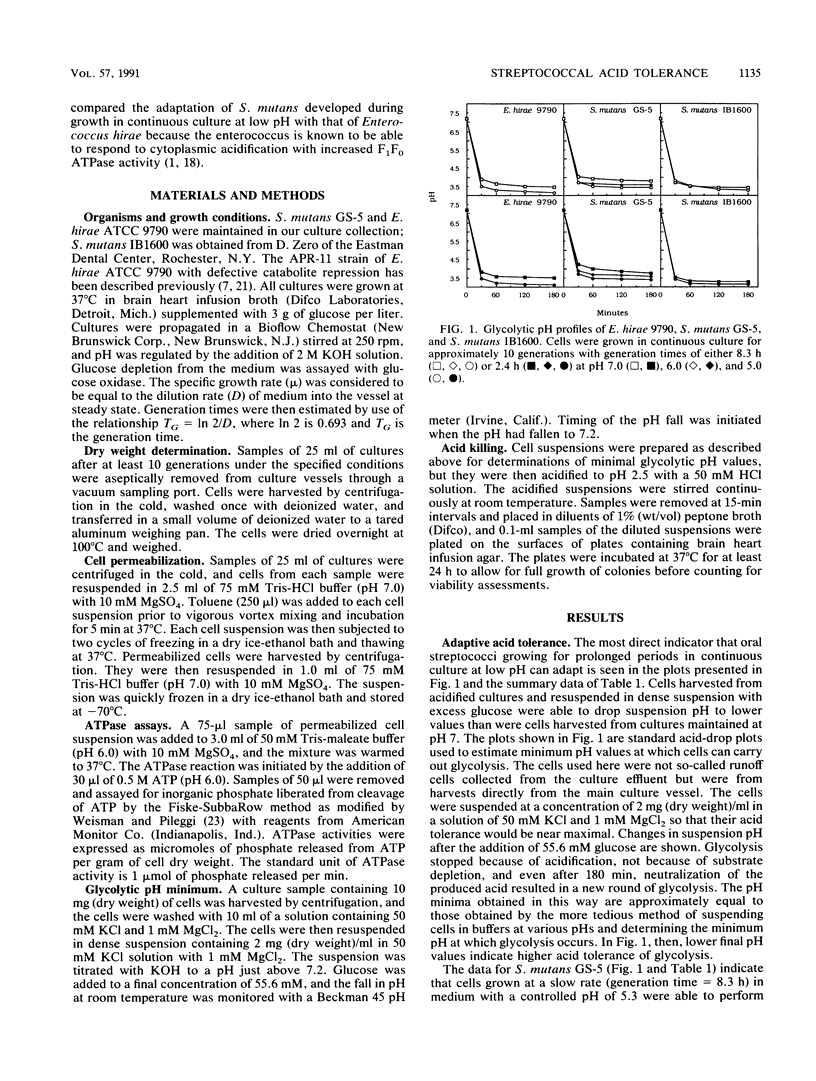
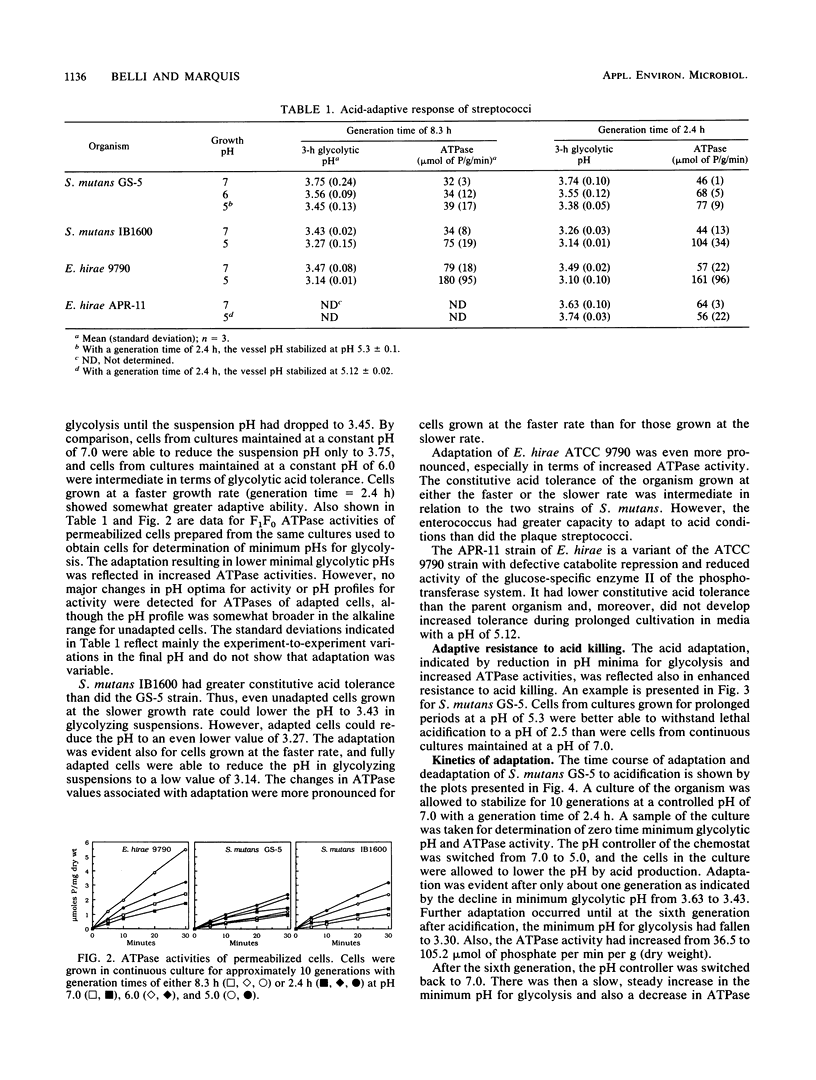
Streptococcus mutans GS-5 and IB1600 adapted to growth in acidic environments in continuous culture at slow (generation time = 8.3 h) or fast (generation time = 2.4 h) rates of growth in complex medium with a restricted glucose supply. The extent of adaptation was indicated by changes in minimum pH values attained by harvested cells suspended in dense suspensions with excess glucose and by increased levels of ATPase activity assayed in permeabilized cells. Also, adapted cells better withstood potentially lethal acidification. Cells harvested from cultures growing at pH values close to 5 reduced suspension pH to lower values than cells from cultures maintained at pH 7. Cells from pH 6 cultures were intermediate. The IB1600 strain had a higher level of constitutive acid resistance than the GS-5 strain and also was better able to adapt to growth in acidified media. Both had less adaptive capacity than Enterococcus hirae ATCC 9790. Adaptation occurred rapidly, mainly within a single generation in continuous culture, while deadaptation occurred more slowly over multiple generations. The capacity of S. mutans to adapt to acid conditions is likely to be important in the ecology of dental plaque and also for the cariogenicity of the organism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams A., Jensen C. Altered expression of the H+ ATPase in Streptococcus faecalis membranes. Biochem Biophys Res Commun. 1984 Jul 18;122(1):151–157. doi: 10.1016/0006-291x(84)90452-2. [DOI] [PubMed] [Google Scholar]
- Abrams A., Morris D., Jensen C. Chymotryptic conversion of bacterial membrane ATPase to an active form with modified alpha chains and defective membrane binding properties. Biochemistry. 1976 Dec 14;15(25):5560–5566. doi: 10.1021/bi00670a021. [DOI] [PubMed] [Google Scholar]
- Bender G. R., Marquis R. E. Membrane ATPases and acid tolerance of Actinomyces viscosus and Lactobacillus casei. Appl Environ Microbiol. 1987 Sep;53(9):2124–2128. doi: 10.1128/aem.53.9.2124-2128.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender G. R., Sutton S. V., Marquis R. E. Acid tolerance, proton permeabilities, and membrane ATPases of oral streptococci. Infect Immun. 1986 Aug;53(2):331–338. doi: 10.1128/iai.53.2.331-338.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden G. H., Hamilton I. R. Competition between Streptococcus mutans and Lactobacillus casei in mixed continuous culture. Oral Microbiol Immunol. 1989 Jun;4(2):57–64. doi: 10.1111/j.1399-302x.1989.tb00100.x. [DOI] [PubMed] [Google Scholar]
- Bowden G. H., Hamilton I. R. Environmental pH as a factor in the competition between strains of the oral streptococci Streptococcus mutans, S. sanguis, and "S. mitior" growing in continuous culture. Can J Microbiol. 1987 Sep;33(9):824–827. doi: 10.1139/m87-143. [DOI] [PubMed] [Google Scholar]
- Campbell J., 3rd, Bender G. R., Marquis R. E. Barotolerant variant of Streptococcus faecalis with reduced sensitivity to glucose catabolite repression. Can J Microbiol. 1985 Jul;31(7):644–650. doi: 10.1139/m85-121. [DOI] [PubMed] [Google Scholar]
- Casiano-Colón A., Marquis R. E. Role of the arginine deiminase system in protecting oral bacteria and an enzymatic basis for acid tolerance. Appl Environ Microbiol. 1988 Jun;54(6):1318–1324. doi: 10.1128/aem.54.6.1318-1324.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraso P., Cid A., Serrano R. Tight control of the amount of yeast plasma membrane ATPase during changes in growth conditions and gene dosage. FEBS Lett. 1987 Nov 16;224(1):193–197. doi: 10.1016/0014-5793(87)80446-5. [DOI] [PubMed] [Google Scholar]
- Eraso P., Gancedo C. Activation of yeast plasma membrane ATPase by acid pH during growth. FEBS Lett. 1987 Nov 16;224(1):187–192. doi: 10.1016/0014-5793(87)80445-3. [DOI] [PubMed] [Google Scholar]
- Foster J. W., Hall H. K. Adaptive acidification tolerance response of Salmonella typhimurium. J Bacteriol. 1990 Feb;172(2):771–778. doi: 10.1128/jb.172.2.771-778.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F., Epps H. M. The effect of the pH of the medium during growth on the enzymic activities of bacteria (Escherichia coli and Micrococcus lysodeikticus) and the biological significance of the changes produced. Biochem J. 1942 Sep;36(7-9):600–618. doi: 10.1042/bj0360600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyde M., Portalier R. Acid shock proteins of Escherichia coli. FEMS Microbiol Lett. 1990 May;57(1-2):19–26. doi: 10.1016/0378-1097(90)90406-g. [DOI] [PubMed] [Google Scholar]
- Hickey E. W., Hirshfield I. N. Low-pH-induced effects on patterns of protein synthesis and on internal pH in Escherichia coli and Salmonella typhimurium. Appl Environ Microbiol. 1990 Apr;56(4):1038–1045. doi: 10.1128/aem.56.4.1038-1045.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D. J., Brusilow W. S., Simoni R. D. In vivo evidence for the role of the epsilon subunit as an inhibitor of the proton-translocating ATPase of Escherichia coli. J Bacteriol. 1984 Dec;160(3):1055–1060. doi: 10.1128/jb.160.3.1055-1060.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky D. J., Herman P. K., Emr S. D. The fungal vacuole: composition, function, and biogenesis. Microbiol Rev. 1990 Sep;54(3):266–292. doi: 10.1128/mr.54.3.266-292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Suzuki T., Kinoshita N., Unemoto T. Amplification of the Streptococcus faecalis proton-translocating ATPase by a decrease in cytoplasmic pH. J Bacteriol. 1984 Jun;158(3):1157–1160. doi: 10.1128/jb.158.3.1157-1160.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Suzuki T., Unemoto T. Streptococcal cytoplasmic pH is regulated by changes in amount and activity of a proton-translocating ATPase. J Biol Chem. 1986 Jan 15;261(2):627–630. [PubMed] [Google Scholar]
- Loesche W. J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986 Dec;50(4):353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis R. E., Bender G. R. Isolation of a variant of Streptococcus faecalis with enhanced barotolerance. Can J Microbiol. 1980 Mar;26(3):371–376. doi: 10.1139/m80-060. [DOI] [PubMed] [Google Scholar]
- Ten Cate J. M. In vitro studies on the effects of fluoride on de- and remineralization. J Dent Res. 1990 Feb;69(Spec No):614–636. doi: 10.1177/00220345900690S120. [DOI] [PubMed] [Google Scholar]


