Abstract
Production of the plant hormone indole-3-acetic acid (IAA) is widespread among plant-associated microorganisms. The non-gall-forming phytopathogen Erwinia chrysanthemi 3937 (strain Ech3937) possesses iaaM (ASAP16562) and iaaH (ASAP16563) gene homologues. In this work, the null knockout iaaM mutant strain Ech138 was constructed. The IAA production by Ech138 was reduced in M9 minimal medium supplemented with l-tryptophan. Compared with wild-type Ech3937, Ech138 exhibited reduced ability to produce local maceration, but its multiplication in Saintpaulia ionantha was unaffected. The pectate lyase production of Ech138 was diminished. Compared with wild-type Ech3937, the expression levels of an oligogalacturonate lyase gene, ogl, and three endopectate lyase genes, pelD, pelI, and pelL, were reduced in Ech138 as determined by a green fluorescent protein-based fluorescence-activated cell sorting promoter activity assay. In addition, the transcription of type III secretion system (T3SS) genes, dspE (a putative T3SS effector) and hrpN (T3SS harpin), was found to be diminished in the iaaM mutant Ech138. Compared with Ech3937, reduced expression of hrpL (a T3SS alternative sigma factor) and gacA but increased expression of rsmA in Ech138 was also observed, suggesting that the regulation of T3SS and pectate lyase genes by IAA biosynthesis might be partially due to the posttranscriptional regulation of the Gac-Rsm regulatory pathway.
Erwinia chrysanthemi is an opportunistic phytopathogen that causes soft rot, wilt, and blight diseases on a wide range of plant host species (18, 39). Several virulence determinants have been discovered in E. chrysanthemi including the type III secretion system (T3SS) and well-studied extracellular enzymes such as pectate lyase and pectinase (35, 97).
In E. chrysanthemi, the pectinases (Pel), which are secreted through the type II secretion system, attack the plant cell wall pectin and lead to the loss of plant cell wall structural integrity (1, 44, 91, 93, 98, 99). The production of pectinases is induced by pectin and its derivatives including polygalacturonate (PGA) and is tightly regulated by environmental conditions (5, 39, 40). Several transcriptional regulators, including Fur, HNS, KdgR, PecS, PecT, cyclic AMP receptor protein, ExpR, and Pir, have been shown to modulate the expression of the genes encoding Pel enzymes (12, 13, 19, 20, 29, 39, 67, 68, 70, 74, 83, 84, 85, 86, 95). In addition, several interconnections between these regulatory systems have been observed (39, 67, 69, 71, 72, 73, 74). Consequently, this complex regulatory network allows E. chrysanthemi to use pectin as a sole carbon and energy source for growth as well as to finely adjust the synthesis of its virulence factors during pathogenesis.
The T3SS is considered one of the major pathogenic factors in many bacterial pathogens (14, 21, 31, 32, 35, 103, 104). Recently, a T3SS regulatory pathway of strain Ech3937 was elucidated (105). Similarly to Pseudomonas syringae and Erwinia and Pantoea spp., the T3SS of Ech3937 is regulated by HrpX/HrpY, a two-component signal transduction system (TCSTS). The HrpX/HrpY system activates the enhancer HrpS. The HrpS protein activates the expression of an alternative sigma factor, HrpL (101, 105), and HrpL further activates the expression of genes encoding T3SS effectors such as DspE, structural components such as HrpA, and harpins such as HrpN.
Ech3937 also possesses an Rsm system, which plays a critical role in gene expression and has a profound effect on bacterial metabolism and behavior in many prokaryotic species. RsmA, rsmB, and RsmC are the major components of this global regulatory system. RsmA is a small RNA-binding protein that acts by repressing translation and by shortening the half-life of the mRNA species (24). rsmB is an untranslated regulatory RNA that binds RsmA and neutralizes its negative regulatory effect by forming an inactive ribonucleoprotein complex (24, 57). RsmC controls the production of RsmA and rsmB RNA by positively regulating rsmA and negatively controlling rsmB (26). The Rsm system has been reported to control the production of different extracellular enzymes like pectinases, proteases, and cellulases and secondary metabolites such as phytohormones, antibiotics, pigments, and polysaccharides (2, 9, 10, 15, 16, 25, 26, 36, 37, 46, 49, 53, 55, 57, 58, 65, 66, 80, 90, 100).
TCSTSs are used by organisms to respond to environmental stimuli and adapt to different environmental conditions. The TCSTS GacS/GacA has been reported to play important roles in various biological functions (17, 23, 38, 82). GacS is the putative sensor kinase, and GacA is the response regulator. GacS is suggested to activate GacA, and the activated GacA works as a transcriptional activator and further activates targeted genes. Many virulence factors including pectate lyase, exoprotease, and tabtoxin and syringomycin production are found to be regulated by GacS/GacA homologues in phytopathogens (17, 23). In Pseudomonas syringae pv. tomato, GacA acted as a master regulator of controlling regulatory RNA rsmB, several transcriptional activators, and alternative sigma factors (17). In Erwinia carotovora subsp. carotovora, GacA has been reported to stimulate the transcription of the pectate lyase, polygalacturonase, cellulase, and T3SS genes through the positive regulation of GacA on rsmB (14, 23, 42).
Auxin regulates almost every aspect of plant growth and development in various biological processes including cell division, elongation, and differentiation; root initiation and elongation; vascular system patterning; somatic embryogenesis; apical dominance; flower development; fruit ripening; and tropisms (28, 46, 48, 106). In the plant, indole-3-acetic acid (IAA) can be derived from either of two tryptophan-independent pathways, which may utilize indole-3-glycerol phosphate or indole as a precursor, or four tryptophan-dependent pathways including the indole-3-pyruvic acid (IPyA) pathway, the indole-3-acetonitrile pathway, the indole-3-acetaldoxime pathway, and the tryptamine pathway (41, 106). IAA can be conjugated to amino acids, sugars, and carbohydrates. IAA conjugates have been implicated in several important plant processes including IAA storage, transport, protection from enzymatic destruction, and targeting of the IAA for catabolism. The bioactive form of IAA is believed to be free IAA. Plants maintain the IAA concentration by a complex network of pathways through the interplay of IAA biosynthesis, conjugate formation, hydrolysis, and irreversible oxidation (11, 75).
Similar to plant IAA production, microorganisms also possess several different IAA biosynthetic pathways. The metabolic routes are classified in terms of their intermediates as the indole-3-acetamide (IAM), IPyA, indole-3-acetonitrile, and tryptamine pathways (22). One major route, the IAM pathway, is employed mostly by pathogenic bacteria including the gall-forming bacterium Pseudomonas syringae pv. savastanoi. First, oxidative decarboxylation of tryptophan leading to indole-3-acetamide is catalyzed by IaaM (tryptophan 2-monooxygenase). The conversion of indole-3-acetamide to IAA is catalyzed by IaaH (indole-3-acetamide hydrolase) (l-tryptophan→IAM→IAA) (Fig. 1) (77). Another common pathway, the IPyA pathway (Fig. 1), is the major IAA biosynthetic pathway used by plant growth-promoting bacteria including Pseudomonas putida GR12-2 (78). In many cases, a single bacterial strain may possess more than one pathway (59). The IAM pathway is involved primarily in gall formation, and the IPyA pathway enhances bacterial epiphytic fitness (4, 7, 8, 30, 60, 94).
FIG. 1.
IAM and IPyA metabolic routes of IAA biosynthetic pathways. IaaM, tryptophan-2-monooxygenase; IaaH, indole-3-acetamide hydrolase; IpdC, indole-3-pyruvate decarboxylase.
Although the role of IAA biosynthesis by microorganisms is not fully understood, IAA provides bacteria with a mechanism to influence plant growth by supplementing the host plant's endogenous pool of auxin (3, 6, 78, 96, 102). In several cases, production of IAA by pathogenic bacteria is a major pathogenicity determinant in gall- and knot-forming bacterial species. Recently, IAA has been shown to inhibit the growth of plant-associated pathogens (56). IAA was identified as a signature molecule for Agrobacterium transformation by down-regulating the expression of virA gene through the competition with the TCSTS VirA/VirG inducing signal acetosyringone after the transformation of T-DNA into the host by the bacterium (56).
The recently sequenced E. chrysanthemi 3937 genome revealed that the bacterium contains a complete set of IAA biosynthesis genes in the IAM pathway (unpublished results). Although the iaaM homologue of Ech3937 was discovered to be up-regulated in plant hosts (104), the ability of the bacterium to produce IAA and the role of IAA biosynthesis in Ech3937 pathogenesis are still unknown. In this study, we demonstrate that Ech3937 possesses the IAA biosynthesis gene homologues iaaM (ASAP16562) and iaaH (ASAP16563) and has the capacity to produce IAA through the IAM pathway. The expression of T3SS and pectinase genes under the wild-type Ech3937 and iaaM mutant background was examined using quantitative reverse transcription-PCR (qRT-PCR) and green fluorescent protein (GFP)-based fluorescence-activated cell sorting (FACS) promoter activity assays. Finally, the positive role of IAA biosynthesis for T3SS and exoenzymes through the Gac-Rsm posttranscriptional regulatory pathway was suggested in this work.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and chemicals.
The bacterial strains and plasmids used in this study are listed in Table 1. Wild-type Ech3937 and mutant strains were stored at −80°C in 15% glycerol and grown on LB agar and M9 minimal medium (MM) (92). Antibiotics (μg/ml) used were as follows: ampicillin, 100; chloramphenicol, 25; kanamycin, 50; rifampin, 100; spectinomycin, 50; and tetracycline, 25. Primers used for PCR in this report are also listed in Table 1. IAA, IAM, l-tryptophan (Trp), and PGA sodium salt were purchased from Sigma-Aldrich, Inc. (St. Louis, MO).
TABLE 1.
Bacterial strains, plasmids, and primers used in this study
| Bacterial strain, plasmid, or primer | Characteristic(s)a or sequenceb (5′ to 3′) | Reference or source |
|---|---|---|
| Bacterial strains | ||
| Ech3937 | E. chrysanthemi 3937 wild type | N. Hugouvieux- Cotte-Pattat |
| Ech138 | iaaM homologue (ASAP16562) null knockout mutant; Kmr | This work |
| Ech139 | iaaM and iaaH (ASAP16562 and ASAP16563) double knockout mutant; Kmr | This work |
| Ech138(pLiaaM) | Ech138 containing pLiaaM; Kmr Spr | This work |
| Ech138(pLiaaMH) | Ech138 containing pLiaaMH; Kmr Spr | This work |
| Ech139(pLiaaMH) | Ech139 containing pLiaaMH; Kmr Spr | This work |
| Plasmids | ||
| pCL1920 | Low-copy-number vector; Spr | 50 |
| pRK415 | Low-copy-number vector; Tcr | 47 |
| pGEMT-Easy | PCR cloning vector; Apr | Promega |
| pGΔiaaM | pGEM T-Easy derivative with a 3-kb iaaM deletion construct PCR fragment; Apr | This work |
| pΔiaaM | pRK415 derivative with a 3-kb EcoRI fragment from pGΔiaaM; Tcr | This work |
| pGΔiaaMH | pGEM T-Easy derivative with a 3-kb iaaMH deletion construct PCR fragment; Apr | This work |
| pΔiaaMH | pRK415 derivative with a 3-kb EcoRI fragment from pGΔiaaMH; Tcr | This work |
| pTiaaM | pGEM T-Easy derivative with a 2.4-kb PCR fragment; iaaM+ Apr | This work |
| pTiaaMH | pGEM T-Easy derivative with a 3.7-kb PCR fragment; iaaM+iaaH+ Apr | This work |
| pIaaM | pCL1920 derivative with a 2.4-kb SacI-KpnI fragment from pTiaaM; iaaM+ Spr | This work |
| pIaaMH | pCL1920 derivative with a 3.7-kb SacI-KpnI fragment from pTiaaMH; iaaM+iaaH+ Spr | This work |
| PdspE | pProbe-AT derivative with PCR fragment containing dspE promoter region; Apr | 79 |
| PhrpL | pProbe-AT derivative with PCR fragment containing hrpL promoter region; Apr | This work |
| PhrpN | pProbe-AT derivative with PCR fragment containing 396-bp hrpN promoter region; Apr | This work |
| PgacA | pProbe-AT derivative with PCR fragment containing 788-bp gacA promoter region; Apr | This work |
| PrsmA | pProbe-AT derivative with PCR fragment containing 424-bp rsmA promoter region; Apr | This work |
| PrsmC | pProbe-AT derivative with PCR fragment containing 870-bp rsmC promoter region; Apr | This work |
| Pogl | pProbe-AT derivative with PCR fragment containing 534-bp ogl promoter region; Apr | This work |
| PpelD | pProbe-AT derivative with PCR fragment containing pelD promoter region; Apr | 79 |
| PpelI | pProbe-AT derivative with PCR fragment containing 1,145-bp pelI promoter region; Apr | This work |
| PpelL | pProbe-AT derivative with PCR fragment containing 609-bp pelL promoter region; Apr | This work |
| Primers | ||
| iaaM_A_Km | AGAGCTCTCTAGAGGATCCAAAAACAGCGGGTCTGTATTGCC | This work |
| iaaM_B_Km | GGGACTCTGGGGTTCGAAATCTAGACAACACAAGGCACTGAATTGGCTA | This work |
| iaaM_C_Km | CCAGTAGCTGACATTCATCCCTCGAGGGTTGTGTCCAGACTATTAGGTTTC | This work |
| iaaM_D_Km | TGGTACCCTCGAGAAGCTTGTTTAAGTGACGGCACAGCAG | This work |
| iaaMH_C_Km | CCAGTAGCTGACATTCATCCCTCGAGGCCACCCGTATAGAAACCATCA | This work |
| iaaMH_D_Km | TGGTACCCTCGAGAAGCTTGCATGCAATAGCAATCAGAGAGG | This work |
| iaaM_F | AGAGCTCTCTAGAGGATCCTCACCGCCGCTGGATGACTA | This work |
| iaaM_R | TGGTACCCTCGAGAAGCTTCGATTAACCATGCCACTCTTGC | This work |
| phrpN_F | CGATACCTACCCGCAAGTGA | This work |
| phrpN_R | TGGAACCCAGGGATGACGT | This work |
| phrpL_F | CTGTTTCTGGTTCGGGTCGGT | This work |
| phrpL_R | GCCACTTCCAACGCATCGTC | This work |
| Pogl_F | ACATAAAGCATCAACTGGAGC | This work |
| Pogl_R | CAGATAACATCGGGAGGAGT | This work |
| PpelI_F | GCGTGGAAAAGATGCTGGGATA | This work |
| PpelI_R | TTGGGCGGCGAATGAAGG | This work |
| PpelL_F | ATGCGGTAATGCGGGGAT | This work |
| PpelL_R | GGCCAGAACTGATGTACTGT | This work |
| PgacA_F | TGTTGTTCATAGCGTCTCCTG | This work |
| PgacA_R | CACCATCTGACCGCATCTT | This work |
| PrsmA_F | AGCAGGCGGCAGTGATGT | This work |
| PrsmA_R | GCCAACTCGACGAGTCAAAAT | This work |
| PrsmC_F | AGAGCTCTGTGAACAGGGGCGCTTAAC | This work |
| PrsmC_R | AGAGCTCGCAATGGTGGCGGGTAT | This work |
Apr, ampicillin resistance; Kmr, kanamycin resistance; Spr, spectinomycin resistance; Tcr, tetracycline resistance.
Restriction sites in primers are in boldface, and barcodes are underlined. Barcodes are random computer-designed DNA sequences which provide a complementary region for overlapping PCR products.
Mutant strain construction.
The iaaM (ASAP16562) deletion mutant Ech138 was constructed by a two-step PCR mutagenesis approach as described previously (103). Two sets of primers, iaaM_A_Km and iaaM_B_Km and primers iaaM_C_Km and iaaM_D_Km, which amplify the upstream and downstream flanking regions, respectively, of the iaaM gene were designed. The left PCR fragment (produced by primers iaaM_A_Km and iaaM_B_Km), right PCR fragment (produced by primers iaaM_C_Km and iaaM_D_Km), and kanamycin cassette were connected at their complementary, overlapping regions and cloned into the SacI and KpnI sites of the pRK415 vector (47), resulting in plasmid pΔiaaM. The pΔiaaM construct was further confirmed by DNA sequencing.
The plasmid construct pΔiaaM was electroporated into Ech3937 competent cells. The Ech3937 cells containing pΔiaaM were grown in LB broth, and the ΔiaaM mutant was selected by resistance to kanamycin and screened by sensitivity to tetracycline. To confirm the mutation, chromosomal DNA was isolated from the putative mutant strains; PCR by primers flanking the target iaaM gene followed by DNA sequencing has been performed to locate the disruption site. An iaaM and iaaH (ASAP16562 and ASAP16563) double deletion mutant, Ech139, was constructed in a similar manner using the primer sets iaaM_A_Km and iaaM_B_Km and iaaMH_C_Km and iaaMH_D_Km to generate the upstream and downstream PCR fragments, respectively.
To construct an iaaM complementary plasmid, a fragment with the iaaM gene and its promoter was PCR amplified using the primer pair of iaaM_F and iaaM_R. The PCR product was ligated into a low-copy-number plasmid vector, pCL1920 (50). The resultant plasmid, pIaaM, was further confirmed by PCR and DNA sequencing with the primer pair of iaaM_F and iaaM_R. The plasmid was then electroporated into the iaaM mutant strain Ech138 for complementation. A similar approach was used to construct a complementary plasmid, pIaaMH, using the primers iaaM_F and iaaMH_D_Km, which contained the iaaM and iaaH genes and the promoter regions. To confirm that pLIaaM and pLIaaMH were reintroduced into the iaaM mutant Ech138, in addition to the plasmid-derived spectinomycin resistance obtained by the iaaM mutant Ech138, the plasmids from the bacterial cells were purified and digested with restriction enzymes. Restriction patterns and sizes of the DNA fragments of the digested plasmids were analyzed.
IAA quantification, exoenzyme, and pectate lyase production assays.
IAA quantification was performed with the Fe-H2SO4 reagent (34, 78). Briefly, bacterial strains were propagated overnight in 5 ml of LB broth and then 50-μl aliquots were transferred into 5 ml of M9 minimal medium supplemented with l-tryptophan. The density of each culture was measured spectrophotometrically at 600 nm, and then the bacterial cells were removed from the culture medium by centrifugation. A 1-ml aliquot of the supernatant was mixed vigorously with 4 ml of Salkowski's reagent (150 ml of concentrated H2SO4, 250 ml of distilled H2O, 7.5 ml of 0.5 M FeCl3·6H2O), and the absorbance at 535 nm was measured with a Cary 100 UV-Vis spectrophotometer (Varian Inc., CA). The concentration of IAA in each culture medium was determined by comparison with a standard curve (78).
Plating and the spectrophotometric assays for activity of Pel were carried out as reported by Matsumoto et al. (61). Briefly, a 30-μl sample of bacterial culture or sonicated culture supernatant (optical density at 600 nm [OD600] of 0.5 [ca. 5 × 108 CFU/ml]) was used in the plate assay, and a 10-μl sample of 0.5-OD600 bacterial culture or sonicated culture supernatant was added into 990 μl pectate lyase reaction buffer (0.1 M Tris-HCl, pH 8.5, 0.05% PGA, 0.1 mM CaCl2) for the spectrophotometric assay.
FACS assays.
The gacA, rsmA, and rsmC genes of Ech3937 were identified based on homologues in E. carotovora (unpublished data). The promoter regions of T3SS genes dspE, and hrpN; pectin catabolic genes ogl, pelD, pelI, and pelL; and regulator genes hrpL, gacA, rsmA, and rsmC of Ech3937 were cloned into a GFP reporter vector, pPROBE-AT, to produce PdspE, PhrpN, Pogl, PpelD, PpelI, PpelL, PhrpL, PgacA, PrsmA, and PrsmC, respectively (Table 1). The promoter activity of Ech3937 and Ech138 carrying the GFP reporter plasmid was examined in MM for T3SS genes and T3SS regulators or in MM supplemented with 1% PGA for pectin catabolic genes and regulators. GFP intensity was determined by FACS (Becton Dickinson, San Jose, CA) as described previously (79). Three types of gene expression parameters were measured, including average GFP fluorescence intensity of total bacterial cells (“Total”), average GFP fluorescence intensity of GFP-expressing bacterial cells (“GFP+ mean”), and GFP-expressing bacterial cells as a percentage of the total bacterial cells (“GFP+%”) (31).
For in planta promoter activity assay, the bacterial strains were grown in LB broth at 28°C. A volume of 1 ml of bacterial suspension from each strain was inoculated into the leaves of the African violet cv. Gauguin and incubated at 28°C. The leaves were sliced into small pieces, placed into centrifuge tubes, and centrifuged at 5,000 rpm for 10 min. The intercellular fluids from leaf tissues that contained bacterial cells were separated by centrifugation. The cell pellets were washed with 1× phosphate-buffered saline as described previously (79). To precisely locate the E. chrysanthemi bacterial cells, the test samples were stained with Baclight Red bacterial stain (Molecular Probes, OR) prior to being run on the flow cytometer. Three biological replicates were performed for each treatment.
RT-PCR and qRT-PCR analysis.
A TRI reagent method (Sigma, St. Louis, MO) was used to isolate total RNA from the bacteria. The isolated RNA was treated with Turbo DNA-free DNase kits (Ambion, Austin, TX), and 0.5 μg of the treated RNA was used as template to synthesize cDNA by using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). For qRT-PCRs to quantify the cDNA level of target genes in different samples, the Opticon 2 system (Bio-Rad) was used to collect data. rplU was used as the internal control to normalize the cDNA input of each sample. The primer pairs used in this study are RplUsF (5′ GCG GCA AAA TCA AGG CTG AAG TCG 3′) and RplUsR (5′ CGG TGG CCA GCC TGC TTA CGG TAG 3′) for rplU, HrpLsF (5′ GAT GAT GCT GCT GGA TGC CGA TGT 3′) and HrpLsR (5′ TGC ATC AAC AGC CTG GCG GAG ATA 3′) for hrpL, dspEf (5′ GAT GGC GGA GCT GAA ATC GTT C 3′) and dspEr (5′ CCT TGC CGG ACC GCT TAT CAT T 3′) for dspE, hrpAf (5′ CAG CAA TGG CAG GCA TGC AG 3′) and hrpAr (5′ CTG GCC GTC GGT GAT TGA GC 3′) for hrpA, and iaaMf (5′ GGC GCG TAA GGC ATG GCA 3′) and iaaMr (5′ GCC ACG GGA CGC CTC C 3′) for iaaM.
Local maceration assay, growth kinetics, and in planta pectate lyase production.
The local leaf maceration assay was carried out as described previously (104). Briefly, wild-type bacterial cells and Ech138 cells were syringe infiltrated in the middle of each symmetric side of the same leaf with approximately 50 μl of a bacterial suspension at the concentration of 106 CFU/ml. Phosphate buffer (50 mM, pH 7.4) was used to suspend the bacterial cells. Three replicate plants with a total of 12 leaves were inoculated. Inoculated plants were kept in growth chambers at 28°C with 95% relative humidity and a photoperiod of 16 h, with regular water misting to provide humid conditions. The area of maceration on plant leaves caused by the bacterial strains was precisely measured with the ASSESS image analysis software (APS Press, The American Phytopathological Society, St. Paul, MN).
Assays for growth kinetics in planta were carried out in African violet cv. Gauguin as described previously (104). Briefly, leaves were syringe infiltrated with approximately 50 μl bacterial suspension at 106 CFU/ml with a 1-ml syringe. Leaf discs (4 mm in diameter) around the maceration area were harvested at different intervals following infiltration and ground in 50 mM phosphate buffer (pH 7.4). The bacterial populations (CFU/cm2) were determined by plating serial dilutions of leaf extracts on LB agar plates. A spectrophotometric assay was used to monitor the pectate lyase production of Ech3937 and Ech138 during the in planta growth. A 10-μl supernatant of the plant juice from African violet leaves inoculated with the bacteria was added into 990 μl Pel reaction buffer, and the Pel production was quantified using the spectrophotometric assay (61). Pectate lyase production was the ratio of the OD230 unit to the log unit of the bacterial population [U/log(CFU/cm2)]. Six leaves from six replicate plants were used in each sampling time for the in planta pectate lyase production and bacterial growth kinetics assays.
RESULTS
Genes ASAP16562 and ASAP16563 of Ech3937 involved in IAA biosynthesis.
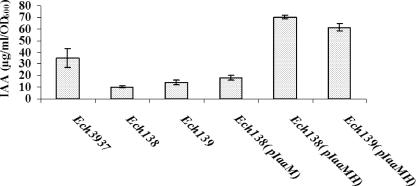
The iaaM (ASAP16562) homologous mutant, Ech138, was constructed using the direct two-step PCR approach (103). An RT-PCR was used to confirm the iaaM mutation. As expected, iaaM mRNA can be detected in wild-type Ech3937 but was not detectable in iaaM mutant Ech138 within 30 PCR cycles (data not shown). There was only basal-level IAA production of cells grown in MM only; the IAA production of Ech3937 required the addition of tryptophan (data not shown). Compared with wild-type Ech3937 in MM supplemented with l-tryptophan at a concentration of 500 μg/ml, the IAA biosynthetic ability of Ech138 was reduced at 48 h of growth (Fig. 2). Similarly, the iaaM and iaaH double mutant, Ech139, also showed reduced IAA biosynthetic ability (Fig. 2). The IAA biosynthesis ability of Ech138 was only partially restored by introducing the iaaM-expressing plasmid, pIaaM, into the mutant (Fig. 2), suggesting that the iaaM mutation may have a polar effect on downstream iaaH. When the iaaM- and iaaH-expressing plasmid, pIaaMH, was introduced into Ech138, the IAA production ability of the mutant was restored (Fig. 2). The IAA production ability of Ech139 was also restored when pIaaMH was introduced into the mutant (Fig. 2). The higher production of IAA in the pIaaMH-complemented strains of Ech138 and Ech139 than in wild-type Ech3937 may be due to the copy number effect of the plasmid.
FIG. 2.
IAA production abilities of Ech3937, Ech138, Ech139, Ech138(pLiaaM), Ech138(pLiaaMH), and Ech139(pLiaaMH) in MM broth supplemented with 500 μg/ml l-tryptophan. IAA was quantified with the Fe-H2SO4 reagent as described previously (34, 78). Bacterial strains were incubated at 28°C with shaking at 200 rpm for 48 h.
IAA biosynthesis of Ech3937 influenced pectate lyase production.
In the MM broth, wild-type Ech3937 and Ech138 had similar growth kinetics (data not shown). Since pectate lyase is one of the key virulence determinants in Ech3937 (43, 88), a spectrophotometric assay was applied to quantify the pectate lyase production (U/OD600) in the Ech138 mutant (61). With a pectate lyase-inducing condition (MM supplemented with 1% PGA), higher extracellular and total pectate lyase activities of Ech3937 was observed in comparison to Ech138 after 12 h of culture. The extracellular and total pectate lyase activities of Ech3937 were 58.5 ± 1.0 and 63.0 ± 0.9, respectively, which were about 163% and 97%, respectively, more than those of Ech138 (22.2 ± 2.4 and 31.9 ± 1.6, respectively). In addition, compared to the pectate lyase production of iaaM mutant Ech138 (7.8 ± 0.7) grown in the IAA biosynthesis-inducing medium of MM supplemented with tryptophan, the pectate lyase production of the complemented strain Ech138(pLiaaMH) was 12.8 ± 1.0, which is almost restored to the wild-type Ech3937 level of 15.2 ± 3.3.
To further confirm the effect of IAA biosynthesis on pectate lyase production by the bacterium, the pectate lyase production by Ech138 in MM, MM supplemented with IAM, or MM supplemented with IAA was quantified using the spectrophotometric assay. Compared with Ech138 grown in MM alone, the production of pectate lyase by Ech138 was increased at least twofold in MM supplemented with IAM or IAA (Table 2), which is comparable to the pectate lyase production by wild-type Ech3937 in MM only (Table 2). In addition, compared with Ech3937 grown in MM alone, a higher pectate lyase production in Ech3937 was observed when the bacterial strain was supplemented with IAM or IAA (Table 2).
TABLE 2.
Spectrophotometric quantification of pectate lyase activity of wild-type E. chrysanthemi 3937 (Ech3937) and iaaM deletion mutant (Ech138) in MM, MM supplemented with 10 μg/ml IAM, and MM supplemented with 100 μg/ml IAA
| Treatment | Bacterial strain | Pectate lyase activitya (U/OD600) |
|---|---|---|
| MM | Ech3937 | 6.8 ± 0.7 |
| Ech138 | 3.9 ± 1.2 | |
| MM-IAM | Ech3937 | 21.2 ± 0.3 |
| Ech138 | 10.7 ± 2.5 | |
| MM-IAA | Ech3937 | 12.7 ± 1.3 |
| Ech138 | 7.7 ± 2.6 |
Pectate lyase activity of bacterial strains grown for 48 h in the medium was determined as described previously (61). One unit of Pel activity was equivalent to an OD230 increase of 0.001 in 1 min. The data represent the averages of two experiments with the standard deviations shown.
Since Ech138 exhibited reduced pectate lyase and the supplementation of IAA or IAM increased the pectate lyase production (Table 2), the effect of IAA biosynthesis on the expression of genes involved in pectin degradation of Ech3937 was investigated. The GFP intensities of bacterial cells of Ech3937 and Ech138 carrying the GFP promoter probe reporter plasmids Pogl, PpelD, PpelI, and PpelL were examined using FACS after 12 h of growth in MM supplemented with PGA. The ogl gene encodes an oligogalacturonate lyase, and the pelD, pelI, and pelL genes encode endopectate lyases.
In the pectate lyase-inducing condition, the average GFP fluorescence intensity of bacterial cells of Ech3937(Pogl), Ech3937(PpelD), Ech3937(PpelI), and Ech3937(PpelL) was higher than that of Ech138(Pogl), Ech138(PpelD), Ech138(PpelI), and Ech138(PpelL), respectively, at 12 h postinoculation (Table 3).
TABLE 3.
ogl, pelD, pelI, pelL, gacA, rsmA, and rsmB promoter activities of wild-type E. chrysanthemi 3937 (Ech3937) and iaaM deletion mutant (Ech138) grown in MM supplemented with 1% polygalacturonate
| Gene promoter | Avg fluorescence intensity for bacterial cellsa:
|
|||
|---|---|---|---|---|
| Ech3937
|
Ech138
|
|||
| Total cells | GFP-expressing cells | Total cells | GFP-expressing cells | |
| Pogl | 132 ± 8 | 137 ± 8 | 103 ± 5 | 106 ± 5 |
| PpelD | 1,135 ± 52 | 1,183 ± 40 | 693 ± 81 | 709 ± 89 |
| PpelI | 57 ± 2 | 58 ± 3 | 48 ± 7 | 49 ± 7 |
| PpelL | 60 ± 2 | 62 ± 2 | 45 ± 2 | 46 ± 2 |
| PgacA | 892 ± 16 | 897 ± 17 | 598 ± 33 | 619 ± 40 |
| PrsmA | 273 ± 5 | 279 ± 5 | 368 ± 28 | 373 ± 30 |
| PrsmC | 99 ± 0 | 105 ± 1 | 168 ± 18 | 171 ± 19 |
The promoter activities were compared after 12 h of culture in medium. GFP intensity was determined on gated populations of bacterial cells by flow cytometry. The percentage of cells expressing GFP (GFP+%) is around 95 to 100%. Values (mean fluorescence intensity) are representative of two experiments. Three biological replicates were used in this experiment.
IAA biosynthesis influences the expression of T3SS genes.
To detect the effect of IAA biosynthesis on the expression of T3SS genes of Ech3937, the bacterial cells were grown in MM and the GFP intensities of PdspE and PhrpN in Ech3937 and Ech138 cells were compared. The dspE gene is a putative T3SS effector, and hrpN is a T3SS harpin, a T3SS substrate. A lower GFP intensity of total bacterial cells (total) was observed in Ech138(PdspE) and Ech138(PhrpN) than in Ech3937(PdspE) and Ech3937(PhrpN) (Table 4). In addition, compared with Ech3937(PdspE) and Ech3937(PhrpN), lower average GFP fluorescence intensity of GFP-expressing bacterial cells (GFP+ mean) and a lower percentage of GFP-expressing bacterial cells relative to the total bacterial cells (GFP+%) were observed in Ech138(PdspE) and Ech138(PhrpN), respectively (Table 4).
TABLE 4.
dspE and hrpN promoter activities of wild-type E. chrysanthemi 3937 (Ech3937) and iaaM deletion mutant (Ech138) grown in MM
| Time of culture (h) | Activity for gene and promotera:
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ech3937(PdspE)
|
Ech138(PdspE)
|
Ech3937(PhrpN)
|
Ech138(PhrpN)
|
Ech3937(PhrpL)
|
Ech138(PhrpL)
|
|||||||||||||
| Total | GFP+ mean | GFP+% | Total | GFP+ mean | GFP+% | Total | GFP+ mean | GFP+ % | Total | GFP+ mean | GFP+% | Total | GFP+ mean | GFP+% | Total | GFP+ mean | GFP+% | |
| 6 | 70 ± 7 | 131 ± 1 | 52 ± 5 | 4 ± 0 | 43 ± 7 | 1 ± 0 | 16 ± 4 | 143 ± 2 | 9 ± 3 | 3 ± 0 | 69 ± 23 | 1 ± 0 | 11 ± 0 | 20 ± 1 | 37 ± 1 | 7 ± 0 | 16 ± 0 | 16 ± 0 |
| 12 | 107 ± 2 | 148 ± 2 | 72 ± 1 | 7 ± 0 | 48 ± 1 | 8 ± 1 | 46 ± 6 | 126 ± 4 | 35 ± 6 | 12 ± 0 | 106 ± 5 | 9 ± 1 | 14 ± 1 | 23 ± 1 | 46 ± 2 | 9 ± 0 | 18 ± 0 | 27 ± 1 |
The promoter activities were compared after 6 and 12 h of culture in MM. GFP intensity was determined on gated populations of bacterial cells by flow cytometry. Values (mean fluorescence intensity) are representative of two experiments. Three biological replicates were used in this experiment. “Total” represents the average GFP fluorescence intensity of total bacterial cells, “GFP+ mean” represents the average GFP fluorescence intensity of GFP-expressing bacterial cells, and “GFP+%” represents GFP-expressing bacterial cells as a percentage of the total bacterial cells.
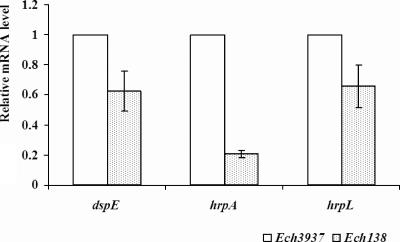
To confirm our FACS results showing the positive effect of IAA biosynthesis on T3SS gene expression, the relative mRNA levels of dspE and hrpN of Ech3937 and Ech138 were examined by qRT-PCR. Compared with Ech3937, a lower amount of dspE and hrpN mRNA was observed in Ech138 (Fig. 3). The levels of dspE and hrpA mRNAs produced by Ech138 were ca. 0.6- and 0.2-fold those of Ech3937, with a P value for the Student t test of less than 0.05 (Fig. 3).
FIG. 3.
Relative levels of dspE, hrpA, and hrpL mRNA in wild-type Ech3937 and iaaM mutant Ech138 grown for 12 h in a minimal medium. The amount of mRNA was examined by a qRT-PCR assay using the Real Master Mix (Eppendorf, Westbury, NY) in different samples. Reactions were run, and data were collected with the Opticon 2 system (Bio-Rad). The rplU gene was used as the internal control to normalize the cDNA input of each sample. Three replicates were used in this experiment, and the Student t test P value is less than 0.05.
Regulatory network of IAA biosynthesis controlling pectate lyase and T3SS gene expression.
Since GacA, RsmA, and RsmC have been reported to regulate the pectate lyase gene expression of Erwinia spp. (16, 23, 24, 26, 42, 52, 57), we examined the regulatory pathway of IAA biosynthesis for pectate lyase gene expression. For this purpose, the GFP intensities of PgacA, PrsmA, and PrsmC in Ech3937 and Ech138 grown in MM supplemented with 1% PGA were compared at 12 h. A higher average GFP fluorescence intensity of total bacterial cells (Total) was observed in Ech138(PrsmA) and Ech138(PrsmC) than in Ech3937(PrsmA) and Ech3937(PrsmC), indicating that IAA biosynthesis down-regulated rsmA and rsmC (Table 3). A lower average GFP fluorescence intensity of total bacterial cells (Total) was observed in Ech138(PgacA) than in Ech3937(PgacA), suggesting that IAA biosynthesis up-regulated gacA in the pectinase-inducing condition.
Similarly, since HrpL has been reported to regulate the T3SS gene expression of Erwinia spp. (14, 23, 26, 64), to further elucidate the regulatory network of IAA biosynthesis on T3SS genes, the GFP intensities of PhrpL in Ech3937 and Ech138 grown in the hrp-inducing MM were compared at 12 h. A higher average GFP fluorescence intensity of total bacterial cells (Total) and a higher percentage of GFP-expressing bacterial cells (GFP+%) were observed in Ech3937(PhrpL) than in Ech138(PhrpL), indicating that IAA biosynthesis up-regulated hrpL (Table 4). Consistent with the FACS result, the level of hrpL mRNA produced by Ech138 was ca. 0.7-fold that of Ech3937, with a P value for the Student t test of less than 0.05 (Fig. 3).
IAA biosynthesis of Ech3937 influenced the local maceration symptoms but not the growth of the bacterium in plants.
The disruption of IAA biosynthesis reduced the expression of certain pectin catabolism-related genes like ogl, pelD, pelI, and pelL (Table 3), T3SS genes like dspE and hrpN, in vitro (Table 4; Fig. 3). PelD was reported to play a major role in causing maceration in plant tissues (88). To test whether the influence of iaaM on pel gene expression holds true in planta, the transcription of pelD was investigated in planta using the GFP reporter. In addition, the expression of two T3SS genes, dspE and hrpN, was also examined in leaves of Saintpaulia ionantha. A lower GFP intensity of total bacterial cells (Total) and a lower percentage of GFP-expressing bacterial cells (GFP+%) of Ech138 carrying PpelD, PdspE, and PhrpN were observed in African violet than in Ech3937 carrying the same GFP reporter plasmid at 24 h (Table 5).
TABLE 5.
pelD, dspE, and hrpN promoter activities of wild-type E. chrysanthemi 3937 (Ech3937) and iaaM deletion mutant (Ech138) grown in African violet cv. Gauguin (Saintpaulia ionantha)
| Strain with gene promoter | Activity at 24 ha
|
||
|---|---|---|---|
| Total | GFP+ mean | GFP+% | |
| Ech3937(PpelD) | 68 ± 6 | 171 ± 14 | 35 ± 0 |
| Ech138(PpelD) | 23 ± 7 | 142 ± 17 | 12 ± 5 |
| Ech3937(PdspE) | 87 ± 42 | 184 ± 27 | 42 ± 17 |
| Ech138(PdspE) | 15 ± 4 | 111 ± 2 | 9 ± 2 |
| Ech3937(PhrpN) | 65 ± 16 | 239 ± 5 | 26 ± 6 |
| Ech138(PhrpN) | 14 ± 14 | 182 ± 14 | 6 ± 7 |
The promoter activities were compared after 24 h of inoculation. GFP intensity was determined on gated populations of bacterial cells by flow cytometry. “Total” represents the average GFP fluorescence intensity of total bacterial cells, “GFP+ mean” represents the average GFP fluorescence intensity of GFP-expressing bacterial cells, and “GFP+%” represents GFP-expressing bacterial cells as a percentage of the total bacterial cells. Values (mean fluorescence intensity) are representative of two experiments. Three biological replicates were used in this experiment.
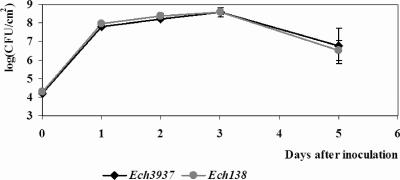
The T3SS genes and the exoenzymes, especially the pectate lyases, are the major virulence determinants in Ech3937. Since the disruption of IAA biosynthesis reduced the production of pectate lyases (Table 2) as well as the expression of certain pectin catabolism-related genes and T3SS genes in vitro (Tables 3 and 4; Fig. 3) and in planta (Table 5), the local maceration and population kinetics of wild-type Ech3937 and Ech138 strains in leaves of S. ionantha were further investigated in order to examine the influence of IAA biosynthesis on bacterial pathogenicity. Compared with wild-type Ech3937 (318 mm2), a significant reduction in the area of maceration of S. ionantha caused by Ech138 (176 mm2) (P = 0.002 by paired-sample t test) was observed at 48 h postinoculation. Meanwhile, a lower pectate lyase activity (U/log [CFU/cm2]) was observed in plant leaves inoculated with Ech138 (1.0 ± 0.3) than in Ech3937 (2.2 ± 0.1) after 3 days postinoculation. Interestingly, the Ech138 strain had in planta multiplication abilities similar to those of wild-type Ech3937 in S. ionantha within 5 days postinoculation (Fig. 4).
FIG. 4.
Population kinetics of wild-type Ech3937 and iaaM mutant Ech138 in African violet cv. Gauguin (Saintpaulia ionantha). Leaves were inoculated with a 50-μl bacterial suspension at 106 CFU/ml. Six leaves from three replicate plants were used at each sampling time for each bacterial strain for the population kinetics, with standard deviations shown.
DISCUSSION
In microorganisms, the IAM pathway genes are generally plasmid borne, and the IPyA pathway genes are chromosomally encoded. For example, iaaM and iaaH genes are borne on a plasmid designated pIAA in P. syringae pv. savastanoi isolated from oleander galls (33). The IPyA pathway is also commonly distributed in higher plants, and the IAA supply is under stringent regulation (59). In contrast, the presence of the IAM pathway in higher plants is quite rare (45) and may be considered unique to bacteria (77). By utilizing the IAM pathway in plant hosts, the pathogen is able to build up large amounts of IAA for gall formation or to control the free IAA level.
From the E. chrysanthemi genome-sequencing project, no obvious IPyA pathway gene homologue was found in Ech3937 (unpublished results). The iaaM (ASAP16562) and iaaH (ASAP16563) genes of wild-type Ech3937 are chromosomally encoded. The reduction of IAA production in Ech138 and Ech139 demonstrated that the IAM pathway serves as an IAA biosynthesis pathway in this bacterium. In this study, it appears that the IAA production is not totally abolished in Ech138 and Ech139. From the Ech3937 genome sequence, Ech3937 contains a gene (ASAP47082) exhibiting similarity to a nitrilase of Arabidopsis thaliana, which is involved in the IAA synthesis of A. thaliana. A reduction of IAA production was observed in the ASAP47082 mutant, suggesting that, along with the IAM pathway, gene ASAP47082 may also be involved in IAA biosynthesis (data not shown).
IAA biosynthesis by phytopathogenic bacteria may contribute to their ability to survive as epiphytes on plant surfaces (22, 30, 54). In addition, the IAA biosynthetic pathways of bacteria may also be involved in detoxification of tryptophan analogues, suppression of plant disease response gene expression, or inhibition of the plant hypersensitive response to facilitate bacterial invasion (22, 62, 89). Finally, various effects of IAA on plant tissues, including modification of electrochemical proton gradients across the host plasmalemma to promote solute uptake (81), alteration of respiration, protein synthesis in cells and enzyme secretion activities of various cell wall polymers, and the induction of host plant ethylene synthesis, have been reported elsewhere (30).
In this study, we demonstrate that the IAA biosynthesis pathway of Ech3937 modulates the expression of T3SS and pectinase genes of the bacterium. The disruption of the iaaM gene decreased the production of IAA and pectate lyase and the expression of T3SS genes (Fig. 2 and 3; Tables 3, 4, and 5). The addition of the IAA biosynthesis intermediate indoleacetamide (IAM, the product of the IaaM enzyme) as well as end product IAA to the MM growth medium increases the pectate lyase production in the iaaM mutant Ech138 as well as the wild-type Ech3937 (Table 2), further demonstrating that IAA biosynthesis plays a positive regulatory role in pectate lyase production.
GFP reporters have been widely used to evaluate gene activity in several bacteria, and a numerical model was formulated to interpret the relationship between GFP intensity and gene promoter activity (51). In addition, the FACS-based approach has been used to investigate gene expression in bacteria at the single-cell level (27, 87). The promoter probe reporter vector, pPROBE-AT, used in this study produces an extremely stable GFP (63). Once the cells have produced GFP, they are fluorescent at later time points even though the cells have stopped generating GFP. In our work, the GFP intensity and percentage of cells expressing GFP at different time periods can be considered a sum of E. chrysanthemi cells that expressed GFP or are expressing GFP from the initial inoculation point until the cells are harvested (79). Rietsch and Mekalanos (87) recently demonstrated by using FACS analysis that the metabolic state of P. aeruginosa regulates the percentage of cells that are able to induce T3SS gene expression (87). In our study, under a homogenous growth environment (MM), distinct regulatory patterns of the IAA biosynthetic pathway of T3SS gene expression at the single-cell level were observed. Compared with wild-type Ech3937(PdspE) and Ech3937(PhrpN), both the GFP fluorescence intensity of bacterial cells and the percentage of GFP-expressing bacterial cells were reduced in Ech138(PdspE) and Ech138(PhrpN) at 12 h of growth in MM (Table 4). Bacterial intercellular communication, e.g., through biochemical signals, provides a mechanism for the regulation of gene expression resulting in coordinated population behavior. Several bacterial pathogens were discovered to use quorum sensing to regulate genes involved in virulence, such as pectinases, T3SS, and motility. At this stage, it is uncertain whether quorum sensing plays a role in this distinct gene regulation pattern of T3SS genes of Ech3937 among individual cells.
From our in planta assay, a decrease in local leaf maceration of Ech138 in S. ionantha was observed in comparison with wild-type Ech3937 2 days postinoculation. A reduced transcription of pelD and reduced pectate lyase production were also observed in Ech138 in African violet leaves in comparison with the wild-type Ech3937 (Table 5). The smaller local leaf maceration area observed in Ech138 might be due to the reduction of pectate lyase production of the mutant strain. Interestingly, the Ech138 strain did not reduce its growth in planta during the 5 days post-bacterial inoculation (Fig. 3), suggesting that the maceration ability of Ech3937 did not fully correspond to the ability of bacterial multiplication in plant tissues. Palva et al. demonstrated that although pectinases secreted by E. chrysanthemi can disassemble the plant cell wall and make the host tissue more accessible to bacteria, the pectate lyases themselves and the oligogalacturonides released through pectate lyase degradation may also trigger the plant defense responses (76). Consequently, a higher resistance response from the host plant might be encountered in the wild-type bacterium than in Ech138 due to a higher pectinase production during the infection process. A previous report demonstrated that an hrpG (encoding the T3SS gene product presumed to be involved in protein secretion) mutant of Ech3937 had a reduced ability to multiply in African violet leaves (103). Mutation of iaaM of Ech3937 reduced but did not fully eliminate the expression of T3SS and pectinase genes Tables 2, 3, 4, and 5; Fig. 3). The phenotype of the iaaM mutant Ech138 suggested that lower levels of pectinase and T3SS expression are sufficient for the multiplication of the bacterium in S. ionantha leaves during the first 5 days of bacterial invasion.
In this study, the expression of dspE and hrpN is decreased in Ech138. Since a lower promoter activity of hrpL was also observed in Ech138 (Table 4), the decrease of dspE and hrpN transcripts may be partially due to the reduction of HrpL in the mutant at the transcriptional level (Tables 4 and 5). At this time, it is uncertain whether the reduced expression of hrpL in Ech138 is through the HrpX-HrpY-HrpS regulatory pathway. In addition, a lower expression of gacA was observed in Ech138. The lower expression of dspE and hrpN in Ech138 might partially be due to a posttranscriptional regulation of the Gac-Rsm regulatory pathway. Indeed, compared with wild-type Ech3937, a smaller amount of mRNA of rsmB and hrpL in the gacA mutant in comparison with Ech3937 was discovered using qRT-PCR (C.-H. Yang et al., unpublished data). Finally, a greater amount of rsmC and rsmA transcript was observed in Ech138. Since RsmA is reported to negatively control the expression of extracellular enzymes (16), a smaller amount of extracellular enzyme production of Ech138 might be partially due to the higher expression of rsmA in the mutant. In conclusion, our data provide the evidence that the disruption of the iaaM gene has an effect on the local maceration pathogenicity on S. ionantha and interferes with the expression of pel genes and T3SS through the Gac-Rsm regulatory pathway.
Acknowledgments
This work is dedicated to Noel T. Keen, who passed away on 18 April 2002.
We thank M. L. P. Collins for a critical review of the manuscript and Q. Peng for assistance in qRT-PCR work.
This project is supported by grants from a Graduate Research Award from the University of Wisconsin—Milwaukee, by the National Science Foundation (award no. EF-0332163), and by Hatch Project 4605.
Footnotes
Published ahead of print on 22 December 2006.
REFERENCES
- 1.Andro, T., J. P. Chambost, A. Kotoujansky, J. Cattaneo, Y. Bertheau, F. Barras, G. F. Van, and A. Coleno. 1984. Mutants of Erwinia chrysanthemi defective in secretion of pectinase and cellulase. J. Bacteriol. 160:1199-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ang, S., Y. T. Horng, J. C. Shu, P. C. Soo, J. H. Liu, W. C. Yi, H. C. Lai, K. T. Luh, S. W. Ho, and S. Swift. 2001. The role of RsmA in the regulation of swarming motility in Serratia marcescens. J. Biomed. Sci. 8:160-169. [DOI] [PubMed] [Google Scholar]
- 3.Barbieri, P., and E. Galli. 1993. Effect on wheat root development of inoculation with an Azospirillum brasilense mutant with altered indole-3-acetic acid production. Res. Microbiol. 144:69-75. [DOI] [PubMed] [Google Scholar]
- 4.Beattie, G. A., and S. E. Lindow. 1995. The secret life of bacterial pathogens on leaves. Annu. Rev. Phytopathol. 33:145-172. [DOI] [PubMed] [Google Scholar]
- 5.Bourson, C., S. Favey, S. Reverchon, and J. Robert-Baudouy. 1993. Regulation of the expression of a pelA::uidA fusion in Erwinia chrysanthemi and demonstration of the synergistic action of plant extract with polygalacturonate on pectate lyase synthesis. J. Gen. Microbiol. 139:1-9. [DOI] [PubMed] [Google Scholar]
- 6.Brandl, M. T., and S. E. Lindow. 1996. Cloning and characterization of a locus encoding an indolepyruvate decarboxylase involved in indole-3-acetic acid synthesis in Erwinia herbicola. Appl. Environ. Microbiol. 62:4121-4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandl, M. T., and S. E. Lindow. 1998. Contribution of indole-3-acetic acid production to the epiphytic fitness of Erwinia herbicola. Appl. Environ. Microbiol. 64:3256-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandl, M. T., B. Quiñones, and S. E. Lindow. 2001. Heterogeneous transcription of an indoleacetic acid biosynthetic gene in Erwinia herbicola on plant surfaces. Proc. Natl. Acad. Sci. USA 98:3454-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burrowes, E., A. Abbas, A. O'Neill, C. Adams, and F. O'Gara. 2005. Characterisation of the regulatory RNA RsmB from Pseudomonas aeruginosa PAO1. Res. Microbiol. 156:7-16. [DOI] [PubMed] [Google Scholar]
- 10.Burrowes, E., C. Baysse, C. Adams, and F. O'Gara. 2006. Influence of the regulatory protein RsmA on cellular functions in Pseudomonas aeruginosa PAO1, as revealed by transcriptome analysis. Microbiology 152:405-418. [DOI] [PubMed] [Google Scholar]
- 11.Campanella, J. J., J. Ludwig-Mueller, and C. D. Town. 1996. Isolation and characterization of mutants of Arabidopsis thaliana with increased resistance to growth inhibition by indoleacetic acid-amino acid conjugates. Plant Physiol. 112:735-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castillo, A., W. Nasser, G. Condemine, and S. Reverchon. 1998. The PecT repressor interacts with regulatory regions of pectate lyase genes in Erwinia chrysanthemi. Biochim. Biophys. Acta 1442:148-160. [DOI] [PubMed] [Google Scholar]
- 13.Castillo, A., and S. Reverchon. 1997. Characterization of the pecT control region from Erwinia chrysanthemi 3937. J. Bacteriol. 179:4909-4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chatterjee, A., Y. Cui, and A. K. Chatterjee. 2002. Regulation of Erwinia carotovora hrpL(Ecc) (sigma-L(Ecc)), which encodes an extracytoplasmic function subfamily of sigma factor required for expression of the HRP regulon. Mol. Plant-Microbe Interact. 15:971-980. [DOI] [PubMed] [Google Scholar]
- 15.Chatterjee, A., Y. Cui, and A. K. Chatterjee. 2002. RsmA and the quorum-sensing signal, N-[3-oxohexanoyl]-l-homoserine lactone, control the levels of rsmB RNA in Erwinia carotovora subsp. carotovora by affecting its stability. J. Bacteriol. 184:4089-4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chatterjee, A., Y. Cui, Y. Liu, C. K. Dumenyo, and A. K. Chatterjee. 1995. Inactivation of rsmA leads to overproduction of extracellular pectinases, cellulases, and proteases in Erwinia carotovora subsp. carotovora in the absence of the starvation/cell density-sensing signal, N-(3-oxohexanoyl)-l-homoserine lactone. Appl. Environ. Microbiol. 61:1959-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatterjee, A., Y. Cui, H. Yang, A. Collmer, J. R. Alfano, and A. K. Chatterjee. 2003. GacA, the response regulator of a two-component system, acts as a master regulator in Pseudomonas syringae pv. tomato DC3000 by controlling regulatory RNA, transcriptional activators, and alternate sigma factors. Mol. Plant-Microbe Interact. 16:1106-1117. [DOI] [PubMed] [Google Scholar]
- 18.Chatterjee, A. K., and M. P. Starr. 1980. Genetics of Erwinia species. Annu. Rev. Microbiol. 34:645-676. [DOI] [PubMed] [Google Scholar]
- 19.Condemine, G., A. Castillo, F. Passeri, and C. Enard. 1999. The PecT repressor coregulates synthesis of exopolysaccharides and virulence factors in Erwinia chrysanthemi. Mol. Plant-Microbe Interact. 12:45-52. [DOI] [PubMed] [Google Scholar]
- 20.Condemine, G., C. Dorel, N. Hugouvieux-Cotte-Pattat, and J. Robert-Baudouy. 1992. Some of the out genes involved in the secretion of pectate lyases in Erwinia chrysanthemi are regulated by kdgR. Mol. Microbiol. 6:3199-3211. [DOI] [PubMed] [Google Scholar]
- 21.Cornelis, G. R., and F. Van Gijsegem. 2000. Assembly and function of type III secretory systems. Annu. Rev. Microbiol. 54:735-774. [DOI] [PubMed] [Google Scholar]
- 22.Costacurta, A., and J. Vanderleyden. 1995. Synthesis of phytohormones by plant-associated bacteria. Crit. Rev. Microbiol. 21:1-18. [DOI] [PubMed] [Google Scholar]
- 23.Cui, Y., A. Chatterjee, and A. K. Chatterjee. 2001. Effects of the two-component system comprising GacA and GacS of Erwinia carotovora subsp. carotovora on the production of global regulatory rsmB RNA, extracellular enzymes, and harpinEcc. Mol. Plant-Microbe Interact. 14:516-526. [DOI] [PubMed] [Google Scholar]
- 24.Cui, Y., A. Chatterjee, Y. Liu, C. K. Dumenyo, and A. K. Chatterjee. 1995. Identification of a global repressor gene, rsmA, of Erwinia carotovora subsp. carotovora that controls extracellular enzymes, N-(3-oxohexanoyl)-l-homoserine lactone, and pathogenicity in soft-rotting Erwinia spp. J. Bacteriol. 177:5108-5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui, Y., L. Madi, A. Mukherjee, C. K. Dumenyo, and A. K. Chatterjee. 1996. The RsmA- mutants of Erwinia carotovora subsp. carotovora strain Ecc71 overexpress hrpNEcc and elicit a hypersensitive reaction-like response in tobacco leaves. Mol. Plant-Microbe Interact. 9:565-573. [DOI] [PubMed] [Google Scholar]
- 26.Cui, Y., A. Mukherjee, C. K. Dumenyo, Y. Liu, and A. K. Chatterjee. 1999. rsmC of the soft-rotting bacterium Erwinia carotovora subsp. carotovora negatively controls extracellular enzyme and harpinEcc production and virulence by modulating levels of regulatory RNA (rsmB) and RNA-binding protein (RsmA). J. Bacteriol. 181:6042-6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dacheux, D., I. Attree, and B. Toussaint. 2001. Expression of ExsA in trans confers type III secretion system-dependent cytotoxicity on noncytotoxic Pseudomonas aeruginosa cystic fibrosis isolates. Infect. Immun. 69:538-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Estelle, M. 1992. The plant hormone auxin: insight in sight. Bioessays 14:439-444. [DOI] [PubMed] [Google Scholar]
- 29.Expert, D. 1999. Withholding and exchanging iron: interactions between Erwinia spp. and their plant hosts. Annu. Rev. Phytopathol. 37:307-334. [DOI] [PubMed] [Google Scholar]
- 30.Fett, W. F., S. F. Osman, and M. F. Dunn. 1987. Auxin production by plant-pathogenic pseudomonads and xanthomonads. Appl. Environ. Microbiol. 53:1839-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frederick, R. D., M. Ahmad, D. R. Majerczak, A. S. Arroyo-Rodriguez, S. Manulis, and D. L. Coplin. 2001. Genetic organization of the Pantoea stewartii subsp. stewartii hrp gene cluster and sequence analysis of the hrpA, hrpC, hrpN, and wtsE operons. Mol. Plant-Microbe Interact. 14:1213-1222. [DOI] [PubMed] [Google Scholar]
- 32.Frederick, R. D., D. R. Majerczak, and D. L. Coplin. 1993. Erwinia stewartii WtsA, a positive regulator of pathogenicity gene expression, is similar to Pseudomonas syringae pv. phaseolicola HrpS. Mol. Microbiol. 9:477-485. [DOI] [PubMed] [Google Scholar]
- 33.Glass, N. L., and T. Kosuge. 1988. Role of indoleacetic acid-lysine synthetase in regulation of indoleacetic acid pool size and virulence of Pseudomonas syringae subsp. savastanoi. J. Bacteriol. 170:2367-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gordon, S. A., and R. P. Weber. 1951. Colorimetric estimation of indoleacetic acid. Plant Physiol. 26:192-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ham, J. H., Y. Cui, J. R. Alfano, P. Rodriguez-Palenzuela, C. M. Rojas, A. K. Chatterjee, and A. Collmer. 2004. Analysis of Erwinia chrysanthemi EC16 pelE::uidA, pelL::uidA, and hrpN::uidA mutants reveals strain-specific atypical regulation of the Hrp type III secretion system. Mol. Plant-Microbe Interact. 17:184-194. [DOI] [PubMed] [Google Scholar]
- 36.Heeb, S., C. Blumer, and D. Haas. 2002. Regulatory RNA as mediator in GacA/RsmA-dependent global control of exoproduct formation in Pseudomonas fluorescens CHA0. J. Bacteriol. 184:1046-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heeb, S., S. A. Kuehne, M. Bycroft, S. Crivii, M. D. Allen, D. Haas, M. Camara, and P. Williams. 2006. Functional analysis of the post-transcriptional regulator RsmA reveals a novel RNA-binding site. J. Mol. Biol. 355:1026-1036. [DOI] [PubMed] [Google Scholar]
- 38.Heeb, S., C. Valverde, C. Gigot-Bonnefoy, and D. Haas. 2005. Role of the stress sigma factor RpoS in GacA/RsmA-controlled secondary metabolism and resistance to oxidative stress in Pseudomonas fluorescens CHA0. FEMS Microbiol. Lett. 243:251-258. [DOI] [PubMed] [Google Scholar]
- 39.Hugouvieux-Cotte-Pattat, N., G. Condemine, W. Nasser, and S. Reverchon. 1996. Regulation of pectinolysis in Erwinia chrysanthemi. Annu. Rev. Microbiol. 50:213-257. [DOI] [PubMed] [Google Scholar]
- 40.Hugouvieux-Cotte-Pattat, N., H. Dominguez, and J. Robert-Baudouy. 1992. Environmental conditions affect transcription of the pectinase genes of Erwinia chrysanthemi 3937. J. Bacteriol. 174:7807-7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hull, A. K., R. Vij, and J. L. Celenza. 2000. Arabidopsis cytochrome P450s that catalyze the first step of tryptophan-dependent indole-3-acetic acid biosynthesis. Proc. Natl. Acad. Sci. USA 97:2379-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hyytiainen, H., M. Montesano, and E. T. Palva. 2001. Global regulators ExpA (GacA) and KdgR modulate extracellular enzyme gene expression through the RsmA-rsmB system in Erwinia carotovora subsp. carotovora. Mol. Plant-Microbe Interact. 14:931-938. [DOI] [PubMed] [Google Scholar]
- 43.Jafra, S., I. Figura, N. Hugouvieux-Cotte-Pattat, and E. Lojkowska. 1999. Expression of Erwinia chrysanthemi pectinase genes pelI, pelL, and pelZ during infection of potato tubers. Mol. Plant-Microbe Interact. 12:845-851. [Google Scholar]
- 44.Ji, J., N. Hugouvieux-Cotte-Pattat, and J. Robert-Baudouy. 1989. Molecular cloning of the outJ gene involved in pectate lyase secretion by Erwinia chrysanthemi. Mol. Microbiol. 3:285-293. [DOI] [PubMed] [Google Scholar]
- 45.Kawaguchi, M., and K. Syono. 1996. Excessive production of indole-3-acetic acid and its significance in studies of the biosynthesis of this regulator of plant growth and development. Plant Cell Physiol. 37:1043-1048. [DOI] [PubMed] [Google Scholar]
- 46.Kay, E., C. Dubuis, and D. Haas. 2005. Three small RNAs jointly ensure secondary metabolism and biocontrol in Pseudomonas fluorescens CHA0. Proc. Natl. Acad. Sci. USA 102:17136-17141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene 70:191-197. [DOI] [PubMed] [Google Scholar]
- 48.King, J. J., D. P. Stimart, R. H. Fisher, and A. B. Bleecker. 1995. A mutation altering auxin homeostasis and plant morphology in Arabidopsis. Plant Cell 7:2023-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lenz, D. H., M. B. Miller, J. Zhu, R. V. Kulkarni, and B. L. Bassler. 2005. CsrA and three redundant small RNAs regulate quorum sensing in Vibrio cholerae. Mol. Microbiol. 58:1186-1202. [DOI] [PubMed] [Google Scholar]
- 50.Lerner, C. G., and M. Inouye. 1990. Low copy number plasmids for regulated low-level expression of cloned genes in Escherichia coli with blue/white insert screening capability. Nucleic Acids Res. 18:4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leveau, J. J., and S. E. Lindow. 2001. Predictive and interpretive simulation of green fluorescent protein expression in reporter bacteria. J. Bacteriol. 183:6752-6762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liao, C. H., D. E. McCallus, W. F. Fett, and Y. Kang. 1997. Identification of gene loci controlling pectate lyase production and soft-rot pathogenicity in Pseudomonas marginalis. Can. J. Microbiol. 43:425-431. [DOI] [PubMed] [Google Scholar]
- 53.Liaw, S. J., H. C. Lai, S. W. Ho, K. T. Luh, and W. B. Wang. 2003. Role of RsmA in the regulation of swarming motility and virulence factor expression in Proteus mirabilis. J. Med. Microbiol. 52:19-28. [DOI] [PubMed] [Google Scholar]
- 54.Lindow, S. E., and M. T. Brandl. 2003. Microbiology of the phyllosphere. Appl. Environ. Microbiol. 69:1875-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu, M. Y., G. Gui, B. Wei, J. F. Preston III, L. Oakford, U. Yuksel, D. P. Giedroc, and T. Romeo. 1997. The RNA molecule CsrB binds to the global regulatory protein CsrA and antagonizes its activity in Escherichia coli. J. Biol. Chem. 272:17502-17510. [DOI] [PubMed] [Google Scholar]
- 56.Liu, P., and E. W. Nester. 2006. Indoleacetic acid, a product of transferred DNA, inhibits vir gene expression and growth of Agrobacterium tumefaciens C58. Proc. Natl. Acad. Sci. USA 12:4658-4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu, Y., Y. Cui, A. Mukherjee, and A. K. Chatterjee. 1998. Characterization of a novel RNA regulator of Erwinia carotovora ssp. carotovora that controls production of extracellular enzymes and secondary metabolites. Mol. Microbiol. 29:219-234. [DOI] [PubMed] [Google Scholar]
- 58.Ma, W., Y. Cui, Y. Liu, C. K. Dumenyo, A. Mukherjee, and A. K. Chatterjee. 2001. Molecular characterization of global regulatory RNA species that control pathogenicity factors in Erwinia amylovora and Erwinia herbicola pv. gypsophilae. J. Bacteriol. 183:1870-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manulis, S., L. Valinski, Y. Gafni, and J. Hershenhorn. 1991. Indole-3-acetic acid biosynthetic pathways in Erwinia herbicola in relation to pathogenicity on Gypsophila paniculata. Physiol. Mol. Plant Pathol. 39:161-171. [Google Scholar]
- 60.Manulis, S., A. Haviv-Chesner, M. T. Brandl, S. E. Lindow, and I. Barash. 1998. Differential involvement of indole-3-acetic acid biosynthetic pathways in pathogenicity and epiphytic fitness of Erwinia herbicola pv. gypsophilae. Mol. Plant-Microbe Interact. 11:634-642. [DOI] [PubMed] [Google Scholar]
- 61.Matsumoto, H., H. Muroi, M. Umehara, Y. Yoshitake, and S. Tsuyumu. 2003. Peh production, flagellum synthesis, and virulence reduced in Erwinia carotovora subsp. carotovora by mutation in a homologue of cytR. Mol. Plant-Microbe Interact. 16:389-397. [DOI] [PubMed] [Google Scholar]
- 62.Mazzola, M., and F. F. White. 1994. A mutation in the indole-3-acetic acid biosynthesis pathway of Pseudomonas syringae pv. syringae affects growth in Phaseolus vulgaris and syringomycin production. J. Bacteriol. 176:1374-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miller, W. G., J. H. Leveau, and S. E. Lindow. 2000. Improved gfp and inaZ broad-host-range promoter-probe vectors. Mol. Plant-Microbe Interact. 13:1243-1250. [DOI] [PubMed] [Google Scholar]
- 64.Mukherjee, A., Y. Cui, Y. Liu, and A. K. Chatterjee. 1997. Molecular characterization and expression of the Erwinia carotovora hrpNEcc gene, which encodes an elicitor of the hypersensitive reaction. Mol. Plant-Microbe Interact. 10:462-471. [DOI] [PubMed] [Google Scholar]
- 65.Mukherjee, A., Y. Cui, Y. Liu, C. K. Dumenyo, and A. K. Chatterjee. 1996. Global regulation in Erwinia species by Erwinia carotovora rsmA, a homologue of Escherichia coli csrA: repression of secondary metabolites, pathogenicity and hypersensitive reaction. Microbiology 142:427-434. [DOI] [PubMed] [Google Scholar]
- 66.Mulcahy, H., J. O'Callaghan, E. P. O'Grady, C. Adams, and F. O'Gara. 2006. The posttranscriptional regulator RsmA plays a role in the interaction between Pseudomonas aeruginosa and human airway epithelial cells by positively regulating the type III secretion system. Infect. Immun. 74:3012-3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nasser, W., M. L. Bouillant, G. Salmond, and S. Reverchon. 1998. Characterization of the Erwinia chrysanthemi expI-expR locus directing the synthesis of two N-acyl-homoserine lactone signal molecules. Mol. Microbiol. 29:1391-1405. [DOI] [PubMed] [Google Scholar]
- 68.Nasser, W., M. Faelen, N. Hugouvieux-Cotte-Pattat, and S. Reverchon. 2001. Role of the nucleoid-associated protein H-NS in the synthesis of virulence factors in the phytopathogenic bacterium Erwinia chrysanthemi. Mol. Plant-Microbe Interact. 14:10-20. [DOI] [PubMed] [Google Scholar]
- 69.Nasser, W., and S. Reverchon. 2002. H-NS-dependent activation of pectate lyases synthesis in the phytopathogenic bacterium Erwinia chrysanthemi is mediated by the PecT repressor. Mol. Microbiol. 43:733-748. [DOI] [PubMed] [Google Scholar]
- 70.Nasser, W., S. Reverchon, G. Condemine, and J. Robert-Baudouy. 1994. Specific interactions of Erwinia chrysanthemi KdgR repressor with different operators of genes involved in pectinolysis. J. Mol. Biol. 236:427-440. [DOI] [PubMed] [Google Scholar]
- 71.Nasser, W., S. Reverchon, R. Vedel, and M. Boccara. 2005. PecS and PecT coregulate the synthesis of HrpN and pectate lyases, two virulence determinants in Erwinia chrysanthemi 3937. Mol. Plant-Microbe Interact. 18:1205-1214. [DOI] [PubMed] [Google Scholar]
- 72.Nasser, W., J. Robert-Baudouy, and S. Reverchon. 1997. Antagonistic effect of CRP and KdgR in the transcription control of the Erwinia chrysanthemi pectinolysis genes. Mol. Microbiol. 26:1071-1082. [DOI] [PubMed] [Google Scholar]
- 73.Nomura, K., W. Nasser, H. Kawagishi, and S. Tsuyumu. 1998. The pir gene of Erwinia chrysanthemi EC16 regulates hyperinduction of pectate lyase virulence genes in response to plant signals. Proc. Natl. Acad. Sci. USA 95:14034-14039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nomura, K., W. Nasser, and S. Tsuyumu. 1999. Self-regulation of pir, a regulatory protein responsible for hyperinduction of pectate lyase in Erwinia chrysanthemi EC16. Mol. Plant-Microbe Interact. 12:385-390. [DOI] [PubMed] [Google Scholar]
- 75.Ostin, A., M. Kowalyczk, R. P. Bhalerao, and G. Sandberg. 1998. Metabolism of indole-3-acetic acid in Arabidopsis. Plant Physiol. 118:285-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Palva, T. K., K. Holmstrom, P. Heino, and E. T. Palva. 1993. Induction of plant defense response by exoenzymes of Erwinia carotovora ssp. carotovora. Mol. Plant-Microbe Interact. 6:190-196. [Google Scholar]
- 77.Patten, C. L., and B. R. Glick. 1996. Bacterial biosynthesis of indole-3-acetic acid. Can. J. Microbiol. 42:207-220. [DOI] [PubMed] [Google Scholar]
- 78.Patten, C. L., and B. R. Glick. 2002. The role of Pseudomonas putida indoleacetic acid in the development of the host plant root system. Appl. Environ. Microbiol. 68:3795-3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peng, Q., S. Yang, A. O. Charkowski, M.-N. Yap, D. A. Steeber, N. T. Keen, and C.-H. Yang. 2006. Population behavior analysis of dspE and pelD regulation in Erwinia chrysanthemi 3937. Mol. Plant-Microbe Interact. 19:451-457. [DOI] [PubMed] [Google Scholar]
- 80.Pessi, G., F. Williams, Z. Hindle, K. Heurlier, M. T. Holden, M. Camara, D. Haas, and P. Williams. 2001. The global posttranscriptional regulator RsmA modulates production of virulence determinants and N-acylhomoserine lactones in Pseudomonas aeruginosa. J. Bacteriol. 183:6676-6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rausch, T., G. Kahl, and W. Hilgenberg. 1984. Primary action of indole-3-acetic acid in crown gall tumors: increase in solute uptake. Plant Physiol. 75:334-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reimmann, C., C. Valverde, E. Kay, and D. Haas. 2005. Posttranscriptional repression of GacS/GacA-controlled genes by the RNA-binding protein RsmE acting together with RsmA in the biocontrol strain Pseudomonas fluorescens CHA0. J. Bacteriol. 187:276-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reverchon, S., M. L. Bouillant, G. Salmond, and W. Nasser. 1998. Integration of the quorum-sensing system in the regulatory networks controlling virulence factor synthesis in Erwinia chrysanthemi. Mol. Microbiol. 29:1407-1418. [DOI] [PubMed] [Google Scholar]
- 84.Reverchon, S., D. Expert, J. Robert-Baudouy, and W. Nasser. 1997. The cyclic AMP receptor protein is the main activator of pectinolysis genes in Erwinia chrysanthemi. J. Bacteriol. 179:3500-3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reverchon, S., W. Nasser, and J. Robert-Baudouy. 1991. Characterization of kdgR, a gene of Erwinia chrysanthemi that regulates pectin degradation. Mol. Microbiol. 5:2203-2216. [DOI] [PubMed] [Google Scholar]
- 86.Reverchon, S., W. Nasser, and J. Robert-Baudouy. 1994. pecS: a locus controlling pectinase, cellulase and blue pigment production in Erwinia chrysanthemi. Mol. Microbiol. 11:1127-1139. [DOI] [PubMed] [Google Scholar]
- 87.Rietsch, A., and J. J. Mekalanos. 2006. Metabolic regulation of type III secretion gene expression in Pseudomonas aeruginosa. Mol. Microbiol. 59:807-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Robert-Baudouy, J., W. Nasser, G. Condemine, S. Reverchon, S. Schevchik, and N. Hugouvieux-Cotte-Pattat. 2000. Pectic enzymes of Erwinia chrysanthemi, regulation and role in pathogenesis, p. 221-268. In G. Stacey and N. T. Keen (ed.), Plant-microbe interactions, vol. 5. American Phytopathological Society Press, St. Paul, MN. [Google Scholar]
- 89.Robinette, D., and A. Matthysse. 1990. Inhibition by Agrobacterium tumefaciens and Pseudomonas savastanoi of development of the hypersensitive response elicited by Pseudomonas syringae pv. phaseolicola. J. Bacteriol. 172:5742-5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Romeo, T. 1998. Global regulation by the small RNA-binding protein CsrA and the non-coding RNA molecule CsrB. Mol. Microbiol. 29:1321-1330. [DOI] [PubMed] [Google Scholar]
- 91.Russel, M. 1998. Macromolecular assembly and secretion across the bacterial cell envelope: type II protein secretion systems. J. Mol. Biol. 279:485-499. [DOI] [PubMed] [Google Scholar]
- 92.Sambrook, J., and D. W. Russell. 2001. Molecular cloning. A laboratory manual, 3rd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 93.Sandkvist, M., L. O. Michel, L. P. Hough, V. M. Morales, M. Bagdasarian, M. Koomey, V. J. DiRita, and M. Bagdasarian. 1997. General secretion pathway (eps) genes required for toxin secretion and outer membrane biogenesis in Vibrio cholerae. J. Bacteriol. 179:6994-7003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Silverstone, S. E., D. G. Gilchrist, R. M. Bostock, and T. Kosuge. 1993. The 73-kb pIAA plasmid increases competitive fitness of Pseudomonas syringae subspecies savastanoi in oleander. Can. J. Microbiol. 39:659-664. [DOI] [PubMed] [Google Scholar]
- 95.Surgey, N., J. Robert-Baudouy, and G. Condemine. 1996. The Erwinia chrysanthemi pecT gene regulates pectinase gene expression. J. Bacteriol. 178:1593-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Surico, G., L. Comai, and T. Kosuge. 1984. Pathogenicity of strains of Pseudomonas syringae pv. savastanoi and their indoleacetic acid-deficient mutants on olive and oleander. Phytopathology 74:490-493. [Google Scholar]
- 97.Tardy, F., W. Nasser, J. Robert-Baudouy, and N. Hugouvieux-Cotte-Pattat. 1997. Comparative analysis of the five major Erwinia chrysanthemi pectate lyases: enzyme characteristics and potential inhibitors. J. Bacteriol. 179:2503-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thomson, N. R., W. Nasser, S. McGowan, M. Sebaihia, and G. P. Salmond. 1999. Erwinia carotovora has two KdgR-like proteins belonging to the IciR family of transcriptional regulators: identification and characterization of the RexZ activator and the KdgR repressor of pathogenesis. Microbiology 145:1531-1545. [DOI] [PubMed] [Google Scholar]
- 99.Thurn, K. K., and A. K. Chatterjee. 1985. Single-site chromosomal Tn5 insertions affect the export of pectolytic and cellulolytic enzymes in Erwinia chrysanthemi EC16. Appl. Environ. Microbiol. 50:894-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Valverde, C., S. Heeb, C. Keel, and D. Haas. 2003. RsmY, a small regulatory RNA, is required in concert with RsmZ for GacA-dependent expression of biocontrol traits in Pseudomonas fluorescens CHA0. Mol. Microbiol. 50:1361-1379. [DOI] [PubMed] [Google Scholar]
- 101.Wei, Z., J. F. Kim, and S. V. Beer. 2000. Regulation of hrp genes and type III protein secretion in Erwinia amylovora by HrpX/HrpY, a novel two-component system, and HrpS. Mol. Plant-Microbe Interact. 13:1251-1262. [DOI] [PubMed] [Google Scholar]
- 102.Xie, H., J. J. Pasternak, and B. R. Glick. 1996. Isolation and characterization of mutants of the plant growth-promoting rhizobacterium Pseudomonas putida GR12-2 that overproduce indoleacetic acid. Curr. Microbiol. 32:67-71. [Google Scholar]
- 103.Yang, C.-H., M. Gavilanes-Ruiz, Y. Okinaka, R. Vedel, I. Berthuy, M. Boccara, J. W. Chen, N. T. Perna, and N. T. Keen. 2002. hrp genes of Erwinia chrysanthemi 3937 are important virulence factors. Mol. Plant-Microbe Interact. 15:472-480. [DOI] [PubMed] [Google Scholar]
- 104.Yang, S., N. T. Perna, D. A. Cooksey, Y. Okinaka, S. E. Lindow, A. M. Ibekwe, N. T. Keen, and C.-H. Yang. 2004. Genome-wide identification of plant-upregulated genes of Erwinia chrysanthemi 3937 using a GFP-based IVET leaf array. Mol. Plant-Microbe Interact. 17:999-1008. [DOI] [PubMed] [Google Scholar]
- 105.Yap, M.-N., C.-H. Yang, J. D. Barak, and A. O. Charkowski. 2005. The Erwinia chrysanthemi type III secretion system is required for multicellular behavior. J. Bacteriol. 187:639-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao, Y., A. K. Hull, N. R. Gupta, K. A. Goss, J. Alonso, J. R. Ecker, J. Normanly, J. Chory, and J. L. Celenza. 2002. Trp-dependent auxin biosynthesis in Arabidopsis: involvement of cytochrome P450s CYP79B2 and CYP79B3. Genes Dev. 16:3100-3112. [DOI] [PMC free article] [PubMed] [Google Scholar]