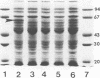
Abstract
A single gene (xylB) encoding both beta-D-xylosidase (EC 3.2.1.37) and alpha-L-arabinofuranosidase (EC 3.2.1.55) activities was identified and sequenced from the ruminal bacterium Butyrivibrio fibrisolvens. The xylB gene consists of a 1.551-bp open reading frame (ORF) encoding 517 amino acids. A subclone containing a 1.843-bp DNA fragment retained both enzymatic activities. Insertion of a 10-bp NotI linker into the EcoRV site within the central region of this ORF abolished both activities. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of cytoplasmic proteins from recombinant Escherichia coli confirmed the presence of a 60,000-molecular-weight protein in active subclones and the absence of this protein in subclones lacking activity. With p-nitrophenyl-beta-D-xylopyranoside and p-nitrophenyl-alpha-L-arabinofuranoside as substrates, the specific activity of arabinosidase was found to be approximately 1.6-fold higher than that of xylosidase. The deduced amino acid sequence of the xylB gene product did not exhibit a high degree of identity with other xylan-degrading enzymes or glycosidases. The xylB gene was located between two incomplete ORFs within the 4,200-bp region which was sequenced. No sequences resembling terminators were found within this region, and these three genes are proposed to be part of a single operon. Based on comparison with other glycosidases, a conserved region was identified in the carboxyl end of the translated xylB gene which is similar to that of glucoamylase from Aspergillus niger.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alff-Steinberger C. Evidence for a coding pattern on the non-coding strand of the E. coli genome. Nucleic Acids Res. 1984 Mar 12;12(5):2235–2241. doi: 10.1093/nar/12.5.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger E., Jones W. A., Jones D. T., Woods D. R. Cloning and sequencing of an endoglucanase (end1) gene from Butyrivibrio fibrisolvens H17c. Mol Gen Genet. 1989 Oct;219(1-2):193–198. doi: 10.1007/BF00261176. [DOI] [PubMed] [Google Scholar]
- Boel E., Hansen M. T., Hjort I., Høegh I., Fiil N. P. Two different types of intervening sequences in the glucoamylase gene from Aspergillus niger. EMBO J. 1984 Jul;3(7):1581–1585. doi: 10.1002/j.1460-2075.1984.tb02014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Béguin P., Cornet P., Aubert J. P. Sequence of a cellulase gene of the thermophilic bacterium Clostridium thermocellum. J Bacteriol. 1985 Apr;162(1):102–105. doi: 10.1128/jb.162.1.102-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotta M. A., Hespell R. B. Proteolytic activity of the ruminal bacterium Butyrivibrio fibrisolvens. Appl Environ Microbiol. 1986 Jul;52(1):51–58. doi: 10.1128/aem.52.1.51-58.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehority B. A. Characterization of several bovine rumen bacteria isolated with a xylan medium. J Bacteriol. 1966 May;91(5):1724–1729. doi: 10.1128/jb.91.5.1724-1729.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehority B. A. Mechanism of isolated hemicellulose and xylan degradation by cellulolytic rumen bacteria. Appl Microbiol. 1968 May;16(5):781–786. doi: 10.1128/am.16.5.781-786.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker R. F., Richards G. N. Hemicellulases: their occurrence, purification, properties, and mode of action. Adv Carbohydr Chem Biochem. 1976;32:277–352. doi: 10.1016/s0065-2318(08)60339-x. [DOI] [PubMed] [Google Scholar]
- Flint H. J., McPherson C. A., Bisset J. Molecular cloning of genes from Ruminococcus flavefaciens encoding xylanase and beta(1-3,1-4)glucanase activities. Appl Environ Microbiol. 1989 May;55(5):1230–1233. doi: 10.1128/aem.55.5.1230-1233.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C. W., Crosby B., Thomas D. Y. Potential for manipulation of the rumen fermentation through the use of recombinant DNA techniques. J Anim Sci. 1986 Jul;63(1):310–325. doi: 10.2527/jas1986.631310x. [DOI] [PubMed] [Google Scholar]
- Grépinet O., Béguin P. Sequence of the cellulase gene of Clostridium thermocellum coding for endoglucanase B. Nucleic Acids Res. 1986 Feb 25;14(4):1791–1799. doi: 10.1093/nar/14.4.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grépinet O., Chebrou M. C., Béguin P. Nucleotide sequence and deletion analysis of the xylanase gene (xynZ) of Clostridium thermocellum. J Bacteriol. 1988 Oct;170(10):4582–4588. doi: 10.1128/jb.170.10.4582-4588.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hespell R. B., O'Bryan-Shah P. J. Esterase activities in Butyrivibrio fibrisolvens strains. Appl Environ Microbiol. 1988 Aug;54(8):1917–1922. doi: 10.1128/aem.54.8.1917-1922.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hespell R. B., Wolf R., Bothast R. J. Fermentation of xylans by Butyrivibrio fibrisolvens and other ruminal bacteria. Appl Environ Microbiol. 1987 Dec;53(12):2849–2853. doi: 10.1128/aem.53.12.2849-2853.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joliff G., Béguin P., Aubert J. P. Nucleotide sequence of the cellulase gene celD encoding endoglucanase D of Clostridium thermocellum. Nucleic Acids Res. 1986 Nov 11;14(21):8605–8613. doi: 10.1093/nar/14.21.8605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lüthi E., Love D. R., McAnulty J., Wallace C., Caughey P. A., Saul D., Bergquist P. L. Cloning, sequence analysis, and expression of genes encoding xylan-degrading enzymes from the thermophile "Caldocellum saccharolyticum". Appl Environ Microbiol. 1990 Apr;56(4):1017–1024. doi: 10.1128/aem.56.4.1017-1024.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannarelli B. M., Evans S., Lee D. Cloning, sequencing, and expression of a xylanase gene from the anaerobic ruminal bacterium Butyrivibrio fibrisolvens. J Bacteriol. 1990 Aug;172(8):4247–4254. doi: 10.1128/jb.172.8.4247-4254.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama H., Fukusaki E., Cabrera Crespo J., Shinmyo A., Okada H. Structure and expression of genes coding for xylan-degrading enzymes of Bacillus pumilus. Eur J Biochem. 1987 Aug 3;166(3):539–545. doi: 10.1111/j.1432-1033.1987.tb13547.x. [DOI] [PubMed] [Google Scholar]
- Quiocho F. A. Carbohydrate-binding proteins: tertiary structures and protein-sugar interactions. Annu Rev Biochem. 1986;55:287–315. doi: 10.1146/annurev.bi.55.070186.001443. [DOI] [PubMed] [Google Scholar]
- Raynal A., Gerbaud C., Francingues M. C., Guerineau M. Sequence and transcription of the beta-glucosidase gene of Kluyveromyces fragilis cloned in Saccharomyces cerevisiae. Curr Genet. 1987;12(3):175–184. doi: 10.1007/BF00436876. [DOI] [PubMed] [Google Scholar]
- Rouvinen J., Bergfors T., Teeri T., Knowles J. K., Jones T. A. Three-dimensional structure of cellobiohydrolase II from Trichoderma reesei. Science. 1990 Jul 27;249(4967):380–386. doi: 10.1126/science.2377893. [DOI] [PubMed] [Google Scholar]
- Sewell G. W., Aldrich H. C., Williams D., Mannarelli B., Wilkie A., Hespell R. B., Smith P. H., Ingram L. O. Isolation and Characterization of Xylan-Degrading Strains of Butyrivibrio fibrisolvens from a Napier Grass-Fed Anaerobic Digester. Appl Environ Microbiol. 1988 May;54(5):1085–1090. doi: 10.1128/aem.54.5.1085-1090.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell G. W., Utt E. A., Hespell R. B., Mackenzie K. F., Ingram L. O. Identification of the Butyrivibrio fibrisolvens xylosidase gene (xylB) coding region and its expression in Escherichia coli. Appl Environ Microbiol. 1989 Feb;55(2):306–311. doi: 10.1128/aem.55.2.306-311.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeri T. T., Lehtovaara P., Kauppinen S., Salovuori I., Knowles J. Homologous domains in Trichoderma reesei cellulolytic enzymes: gene sequence and expression of cellobiohydrolase II. Gene. 1987;51(1):43–52. doi: 10.1016/0378-1119(87)90472-0. [DOI] [PubMed] [Google Scholar]
- Ward O. P., Moo-Young M. Enzymatic degradation of cell wall and related plant polysaccharides. Crit Rev Biotechnol. 1989;8(4):237–274. doi: 10.3109/07388558909148194. [DOI] [PubMed] [Google Scholar]
- Weinstein L., Albersheim P. Structure of Plant Cell Walls: IX. Purification and Partial Characterization of a Wall-degrading Endo-Arabanase and an Arabinosidase from Bacillus subtilis. Plant Physiol. 1979 Mar;63(3):425–432. doi: 10.1104/pp.63.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead T. R., Hespell R. B. The genes for three xylan-degrading activities from Bacteroides ovatus are clustered in a 3.8-kilobase region. J Bacteriol. 1990 May;172(5):2408–2412. doi: 10.1128/jb.172.5.2408-2412.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaguchi M., Roy C., Rollin C. F., Paice M. G., Jurasek L. A fungal cellulase shows sequence homology with the active site of hen egg-white lysozyme. Biochem Biophys Res Commun. 1983 Oct 31;116(2):408–411. doi: 10.1016/0006-291x(83)90537-5. [DOI] [PubMed] [Google Scholar]
- Yasui W., Sumiyoshi H., Ochiai A., Tahara E. Calcium-activated, phospholipid-dependent protein kinase in human gastric mucosa and carcinoma. Jpn J Cancer Res. 1985 Dec;76(12):1168–1173. [PubMed] [Google Scholar]