Abstract
Copines make up a family of soluble, calcium-dependent, membrane binding proteins found in a variety of eukaryotic organisms. In an earlier study, we identified six copine genes in the Dictyostelium discoideum genome and focused our studies on cpnA. Our previous localization studies of green fluorescent protein-tagged CpnA in Dictyostelium suggested that CpnA may have roles in contractile vacuole function, endolysosomal trafficking, and development. To test these hypotheses, we created a cpnA− knockout strain, and here we report the initial characterization of the mutant phenotype. The cpnA− cells exhibited normal growth rates and a slight cytokinesis defect. When placed in starvation conditions, cpnA− cells appeared to aggregate into mounds and form fingers with normal timing; however, they were delayed or arrested in the finger stage. When placed in water, cpnA− cells formed unusually large contractile vacuoles, indicating a defect in contractile vacuole function, while endocytosis and phagocytosis rates for the cpnA− cells were similar to those seen for wild-type cells. These studies indicate that CpnA plays a role in cytokinesis and contractile vacuole function and is required for normal development, specifically in the later stages prior to culmination. We also used real-time reverse transcription-PCR to determine the expression patterns of all six copine genes during development. The six copine genes were expressed in vegetative cells, with each gene exhibiting a distinct pattern of expression throughout development. All of the copine genes except cpnF showed an upregulation of mRNA expression at one or two developmental transitions, suggesting that copines may be important regulators of Dictyostelium development.
Copines are a family of highly conserved, ubiquitously expressed, calcium-dependent membrane binding proteins found in a variety of eukaryotic organisms. Multiple copine homologs are found in Paramecium, Dictyostelium discoideum, Arabidopsis, Caenorhabditis elegans, mice, and humans (9, 10, 27, 47). Although the exact function of copines is not known, a growing body of evidence suggests that copines may mediate an array of cellular processes by conferring calcium regulation to various signaling pathways (21, 43, 44, 47).
Copines are defined by the unique combination of two N-terminal C2 domains responsible for the protein's calcium-dependent membrane binding properties and a protein-binding “A domain” similar to the VWA domain of various extracellular matrix proteins and the extracellular portion of integrins (9, 45). C2 domains are common in eukaryotic kingdoms and are able to interact with diverse ligands, including Ca2+, phospholipids, inositol phosphates, and proteins (32, 42). Most proteins that contain multiple C2 domains appear to regulate vesicular trafficking and exocytosis; examples include synaptotagmin, munc-13, rabphilin, and DOC2. Conversely, single C2 domain proteins generally function as enzymes involved in cell signaling and include protein kinases, lipid-modifying enzymes, and GTPase-activating proteins.
Although copines have two C2 domains, they appear to be different from previously studied proteins with two C2 domains, such as synaptotagmin and DOC2. The two C2 domains of copines are more similar in sequence to the C2 domain of phospholipase C than they are to those of synaptotagmin and DOC2 (9, 31). In addition, the two C2 domains in copines are found in the N terminus, while the C2 domains of the other double C2 domain family members are found in the C terminus. Furthermore, Nakayama et al. (31) found that a neuronally expressed mouse copine localized to cell bodies and dendrites in neurons, whereas other double C2 domain proteins localize to presynaptic nerve terminals. In addition, a copine protein was found to be associated with the nicotinic acetylcholine receptor in C. elegans (16). Therefore, copines may be functionally more related to the single C2 domain proteins involved in signal transduction than to the double C2 domain family involved in vesicle trafficking. The most compelling evidence for the involvement of copines in signaling pathways comes from a study carried out by Tomsig et al. (44) that showed that human copine I regulates signaling from the tumor necrosis factor alpha receptor in human embryonic kidney 293 cells. However, copines may provide a link between signaling and membrane trafficking pathways.
A general hypothesis proposed by Tomsig et al. (43) for how copines may regulate signaling pathways is that specific copines interact with other cellular proteins through their A domains and deliver these target proteins to particular membranes in response to the influx of intracellular calcium through the action of the C2 domains. Identification of more than 20 distinct targets of A domains of human copines I, II, and IV by a yeast two-hybrid screen and the ability of a full-length copine to recruit these ligands to phospholipid surfaces in vitro provided the first evidence in support of this hypothesis (43). Among the proteins that were found to associate with human copine A domains were various regulators of phosphorylation, transcription, ubiquitination, cytoskeletal rearrangement, exocytosis, and mitosis, suggesting that copines carry out many diverse functions. Also in support of this general hypothesis are two studies that show that the A domain alone can act as a dominant negative mutation (26, 44). Overexpression of the A domain by itself would lead to its binding to target proteins, preventing the endogenous copine from binding its targets. Because the A domain alone lacks the C2 domains, the target proteins are not brought to membranes in response to calcium, thereby causing a breakdown of copine function.
Mutant studies with Arabidopsis and C. elegans also suggest that copines may be involved in many different cellular processes, including growth, development, cell death, stress response, pathogen defense, and neuronal signaling. Copine mutants in Arabidopsis have defects in growth and development and increased cell death and disease resistance (19, 21, 22, 47). For C. elegans, GEM4, a copine protein, was found to antagonize the function of GON2, a cation channel required for division of the four postembryonic gonad precursor cells and their development into gonads (8). In addition, inactivation of a copine gene in C. elegans by RNA interference results in nicotine resistance, most likely explained by a reduction in synaptic nicotinic receptor expression (16).
We are studying the function of copine proteins in the simple eukaryote Dictyostelium discoideum. In an earlier study, we identified six copine genes in the Dictyostelium genome (10). All six Dictyostelium copine proteins contain the characteristic two C2 domains followed by a VWA domain, and the amino acid sequence identity among the Dictyostelium copines ranges from 28% between CpnA and CpnF to 60% between CpnB and CpnE. We have focused our studies on one of the copine proteins, CpnA (10). Through double-labeling experiments, we showed that a green fluorescent protein (GFP)-tagged CpnA localized to the plasma membrane and intracellular vacuoles, including organelles of the endolysosomal pathway, phagosomes, and contractile vacuoles in fixed cells. The association of copines with the plasma membrane and particular membrane-bound organelles suggests that copine may have a role in the trafficking of proteins to these membranes. We also observed a transient, rapid, and recurring translocation of GFP-CpnA from cytosol to plasma membrane and vacuoles in a subset of starved cells in vivo. Under starvation conditions, Dictyostelium begin a developmental program to form multicellular fruiting bodies made up of stalk and spore cells (23). Because only a fraction of starved cells displayed the transient GFP-CpnA membrane localization, we hypothesized that CpnA may have a role in development, particularly in the differentiation of cells into specific cell types.
To test these hypotheses and further investigate the function of CpnA, we created a cpnA− knockout strain of Dictyostelium and analyzed this mutant strain for defects in growth, development, contractile vacuole function, endocytosis, and phagocytosis. In addition, we used real-time reverse transcription-PCR (RT-PCR) to determine the developmental expression patterns of all six copine genes, cpnA-cpnF, in Dictyostelium.
MATERIALS AND METHODS
Creation of the cpnA− null mutant.
The DNA cpnA knockout construct was made by PCR subcloning of the 5′ and 3′ flanking sequences of cpnA on either side of the bsr gene in the pBSII plasmid. A 900-bp fragment upstream of the cpnA gene was PCR amplified from genomic DNA by use of the following primers: 5′-AAAATTTTAACTTCTTCATCATCATCATCT-3′ and 5′-GTGACCAAAATAACCTT-3′. A 1,500-bp fragment downstream of the cpnA gene was PCR amplified from genomic DNA by use of the following primers: 5′-GGTGTATCAAATGTATCATTAG-3′ and 5′-CAAATGGTGGAAGGGCTTACC-3′.
These PCR fragments were subcloned into pBSII on either side of the bsr gene. The plasmid was linearized and electroporated into Dictyostelium NC4A2 cells, an axenic strain derived from the wild-type NC4 strain (38). NC4A2 is referred to as “wild type” hereafter. Transformed cells were plated in 96-well plates and grown in HL-5 medium (0.75% proteose peptone, 0.75% thiotone E peptone, 0.5% Oxoid yeast extract, 1% glucose, 2.5 mM Na2HPO4, 8.8 mM KH2PO4, pH 6.5) supplemented with penicillin-streptomycin (Sigma) and blasticidin (ICN). Cells from 20 wells that grew in the presence of blasticidin were passed to larger plates and then screened for CpnA expression by use of Western blotting with polyclonal rabbit antisera raised against a bacterially expressed protein fragment of CpnA. Western blotting was carried out as previously described (10). Sixteen of the 20 clones did not express CpnA, as shown by Western blotting; the remaining 4 were assumed to have undergone nonhomologous recombination to become blasticidin resistant. Three of the clones not expressing CpnA and one blasticidin resistance clone expressing CpnA were selected for PCR screening. For PCR screening of the various transformed cells, the following primers were designed to amplify ∼1 kb of the cpnA gene: 5′-GCTCGAGCAGTAATTGAGAGAGAATATA-3′ and 5′-GGTTATTAATTCTTTTCTAATGATACAT-3′. One of the cpnA− cell clones was transformed with the pTX-GFP/CpnA plasmid and, expression of GFP-CpnA was verified by Western blotting and fluorescence microscopy as described in the work of Damer et al. (10). All cells were stored at −80°C in HL-5 with 10% dimethyl sulfoxide. New cells were thawed and plated every 2 to 3 weeks.
Growth and development assays.
Wild-type cells, nonhomologous recombination blasticidin-resistant control cells, and cpnA− cells were grown in suspension at 19°C in either HL-5 medium or a phosphate buffer (150 mM KH2PO4, 20 mM Na2HPO4, pH 6.1) supplemented with Escherichia coli B/r. Cell densities were estimated at various time points by use of a hemocytometer. For development on filters, 5 × 107 wild-type or cpnA− cells were plated on black filters (catalog no. HABP04700; Millipore), placed on pads in dishes (catalog no. 09-753-53C; Fisher), and allowed to develop for 48 h. For development on bacterial lawns, wild-type and cpnA− cells were diluted into phosphate buffer to 500 cells/ml. Overnight cultures of E. coli B/r in HL-5 were mixed with Dictyostelium cells (5:1) and spread on SM-5 (0.2% proteose peptone 2 [Difco], 0.2% yeast extract [Oxoid], 0.2% glucose, 0.2% MgSO4·7H2O, 0.19% KH2PO4, 1% K2HPO4, pH 6.4) agar plates. Cells were imaged at various time points with an Olympus SZH10 stereo dissecting microscope and photographed at magnifications of ×7 and ×40 with a SPOT Insight color camera and Advanced Spot version 4.0.9 software.
Nucleus-counting assays.
Cells that were growing on plates or in suspension for 6 days were allowed to adhere to coverslips for 15 min in a humid chamber, fixed with 1% formaldehyde in methanol at −10°C for 5 min, washed three times in phosphate-buffered saline (PBS) buffer, incubated with 0.1 μg/ml DAPI (4′,6′-diamidino-2-phenylindole) for 10 min, rinsed with PBS, and mounted on slides. Fixed cells were imaged using epifluorescence optics on a Nikon TE2000 with a 100× objective and a UV filter cube. The number of nuclei in 100 individual cells was counted for each cell type in each assay.
Microscopic analysis of contractile vacuole function.
Cells were placed in glass-bottom dishes and imaged using differential interference contrast on a Nikon TE2000 with a 100× objective, a Cooke Sensicam camera, and Image Pro-Plus software. The number of cells out of every 100 cells containing vacuoles larger than one-fourth of the area of the cell was counted. Initial contractile vacuole counts were done with cells in HL-5. The HL-5 was replaced with water, and contractile vacuole counts were taken at various time points after water replacement. Supplemental movies are 2 frames/s of differential interference contrast (DIC) images taken every 2 seconds (see Movies S1 and S2 in the supplemental material).
Total RNA isolation.
NC4A2 cells were washed four times in PDF starvation buffer (20 mM KCl, 11 mM K2HPO4, 13.2 mM KH2PO4, 1 mM CaCl2, 2.5 mM MgSO4, pH 6.4) by centrifugation at 1,200 rpm for 5 min each, and 2.5 × 107 cells were plated on filters (HABPO4700; Millipore) placed on top of 47-mm petri dishes with pads (09-753-53C; Fisher). Monolayers of dishes were placed in plastic sandwich containers with wet paper towels and allowed to develop at 19°C. Every 2 h for 24 h, the cells from two dishes were washed off the filters by vortexing in PDF buffer and were collected by centrifugation. Total RNA was isolated from these cells by use of an RNeasy minikit according to the manufacturer's instructions for the extraction of cytoplasmic RNA (QIAGEN) and treated with RNase-free DNase (QIAGEN). The amount of RNA was quantified by UV analysis at an A260/280 of >1.9 and visualized on 1.25% SeaKem Gold agarose gels (Cambrex) stained with GelStar nucleic acid stain (Cambrex).
Real-time RT-PCR.
Each RNA sample (50 ng/μl) was reverse transcribed into cDNA by use of a high-capacity cDNA archive kit (Applied Biosystems). RT conditions were alternating cycles of 10 min at 25°C and 120 min at 37°C. Real-time PCR was carried out in 50-μl volumes by use of MicroAmp optical 96-well reaction plates (Applied Biosystems). Each PCR well contained 25.0 μl of 2× TaqMan universal PCR master mix (Applied Biosystems), 17.5 μl RNase free H2O, 5.0 μl cDNA sample, and 2.5 μl of each primer (TaqMan 20X Assays-on-Demand gene expression assay mix; Applied Biosystems). Primers and probe sequences (Table 1) for the six copine genes, two endogenous control genes, and the positive control gene were designed with an Assays-by-Design file builder (Applied Biosystems) using the curated model DNA coding sequence of each gene provided by dictyBase (7). Fluorescence was detected on an Applied Biosystems 7500 real-time PCR system. PCR conditions were 2 min at 50°C, 10 min at 95°C, and 40 cycles of 15 s at 95°C and 1 min at 60°C. Dictyostelium development, RNA isolation, RT, and real-time PCR were performed twice. The real-time RT-PCR samples for each gene at each time point were done in triplicate, and cycle threshold values generated from the reactions were averaged. Cycle threshold values of each gene were normalized to the two endogenous controls and calibrated to an average expression level for the gene being analyzed. The data from the two experimental sets for each gene were averaged and the standard errors calculated.
TABLE 1.
PCR primers and probe sequences
| Gene and primer or probe | Primer or probe sequencea | Gene ID no. |
|---|---|---|
| cpnA | DDB0215368; GenBank:AY332759 | |
| Forward primer | 5′-CAGTATCAGATGCAGTAATTGAGAGAGAAT-3′ | |
| Reverse primer | 5′-TGAATCACCATTACTTGCAGTACAATCA-3′ | |
| Probe | 5′-FAM-ATGGGTGGTTGTCAAATG-NFQ-3′ | |
| cpnB | DDB0216245; GenBank:AY593970 | |
| Forward primer | 5′-CACAACCAATGCCGATACAGTTTT-3′ | |
| Reverse primer | 5′-ACCTGATTTTTTAGCTGCTTTCTTTGC-3′ | |
| Probe | 5′-FAM-ATTGATTAACGGGAATTCT-NFQ-3′ | |
| cpnC | DDB0216239 | |
| Forward primer | 5′-ACTTTGGCCAGAATTACACATGGAA-3′ | |
| Reverse primer | 5′-TGTGCACCAATGCTATCATAATCATAAACTTCA-3′ | |
| Probe | 5′-FAM-CATATCACCCCCATTAAATT-NFQ-3′ | |
| cpnD | DDB0216244 | |
| Forward primer | 5′-CAGATTTACGACAAACACTGACACA-3′ | |
| Reverse primer | 5′-GTACCCGGATTTACCAACTTTCTTTG-3′ | |
| Probe | 5′-FAM-ACTCCAACGCACCACCTC-NFQ-3′ | |
| cpnE | DDB0216242 | |
| Forward primer | 5′-GGTGAAATCACAGATATGGAAGAAACCA-3′ | |
| Reverse primer | 5′-ACCAACACCAACGATAACGATTGAT-3′ | |
| Probe | 5′-FAM-CATCCTCCAAAGCTCC-NFQ-3′ | |
| cpnF | DDB0233133 | |
| Forward primer | 5′-TGAAGTATTTGGATTCGGTGCTGTT-3′ | |
| Reverse primer | 5′-GTGAGGTAAATTGAATCTTTGGTATTGCT-3′ | |
| Probe | 5′-FAM-AATCACACCACTGATACCTG-NFQ-3′ | |
| carA | DDB0185024; GenBank:M21824 | |
| Forward primer | 5′-TGTCAATGGTGGTTTCCCTTGTT-3′ | |
| Reverse primer | 5′-TCTGGTTCTGGTTCTCTTTTTACAATTAACA-3′ | |
| Probe | 5′-FAM-TTGGTTGTGGACTTTATG-NFQ-3′ | |
| cinD | DDB0191304; GenBank:X15385 | |
| Forward primer | 5′-CCATCCCAAGTCAAGCTGTTCTTT-3′ | |
| Reverse primer | 5′-GAGTGGTTTGCCAATTTCTTTTCCT-3′ | |
| Probe | 5′-FAM-AAGCCTTCTCTAATTTTG-NFQ-3′ | |
| rnlA | DDB0216319 | |
| Forward primer | 5′-GGCGCTGGTGAAATAGTAAGTATATTAGA-3′ | |
| Reverse primer | 5′-GGTTACCGCCCCAGTCAAA-3′ | |
| Probe | 5′-FAM-CTACCCGGCATACATTAT-NFQ-3′ |
FAM, 6-carboxyfluorescein; NFQ, nonfluorescent quencher.
Endocytosis, phagocytosis, and exocytosis assays.
For all assays, wild-type and cpnA− cells were harvested from plastic culture dishes and counted, and equal numbers of cells were diluted into HL-5 to a final density of 1 × 106 to 2 × 106 cells/ml. The suspension cultures were allowed to recover on a slow shaker for 16 to 24 h prior to the experiment. Each culture was then separated into two flasks, and control flasks were incubated with 0.02% sodium azide for 30 to 45 min. For endocytosis assays, 20 mg/ml fluorescein isothiocyanate (FITC)-conjugated dextran (FITC-dextran) (molecular weight, 70,000; 490/530; Sigma) was added to each flask. Three-milliliter samples were taken out after 0, 15, 30, and 60 min of incubation and immediately combined with 5 ml of ice-cold HL-5 containing 0.02% sodium azide. Cells were washed three times with cold 0.02% azide-HL-5, kept on ice in the dark until all the samples were collected, and then resuspended in 3 ml of cold Na2HPO4, pH 9.2 plus 2% Triton X-100. For phagocytosis assays, fluorescent beads (FluoSpheres, 1 μm, 505/515; Molecular Probes) were added to the wild-type and cpnA− cultures at the final concentration of 10 μl beads/ml of culture. Samples were taken out after 0, 15, 30, 60, and 90 min of incubation. Cells in each sample were washed four times with cold PBS, resuspended in 3 ml of cold PBS plus 2% Triton X-100, and kept on ice in the dark until all the samples were collected. For exocytosis assays, each culture was incubated for 1 hour with 10 μl beads/ml (FluoSpheres, 1 μm, 505/515; Molecular Probes), after which all cells were washed three times in HL-5 medium and resuspended to the initial concentration in Sörensen phosphate buffer (2 mM Na2HPO4, 14.5 mM KH2PO4, pH 6.2) and 3-ml samples were collected at 0, 20, 40, 60, and 90 min. The cells were pelleted and resuspended in 3 ml of Sorenson's Buffer plus 2% Triton X-100. Both supernatant and the cells were kept on ice in the dark until all the samples were collected.
For all assays, the amount of FITC-dextran or fluorescent beads in each sample was quantified on a Photon Technology International fluorometer. The data were collected using Felix Software, in a time-based mode, and averaged over 20 seconds. The protein content of each sample was determined by Bradford assay using Bio-Rad protein assay dye reagent concentrate, and the data were expressed in terms of μg of FITC-dextran/μl fluorescent beads per mg of protein. The amount of fluorescence in control samples killed with sodium azide was subtracted from data for experimental samples for each time point. Finally, the results were expressed as the percentages of dextran/beads ingested by endocytosis/phagocytosis or released by exocytosis, with the highest reading assigned a value of 100%. For each assay, the data from three experimental trials were averaged and standard errors were calculated.
RESULTS
Creation of the cpnA− knockout strain.
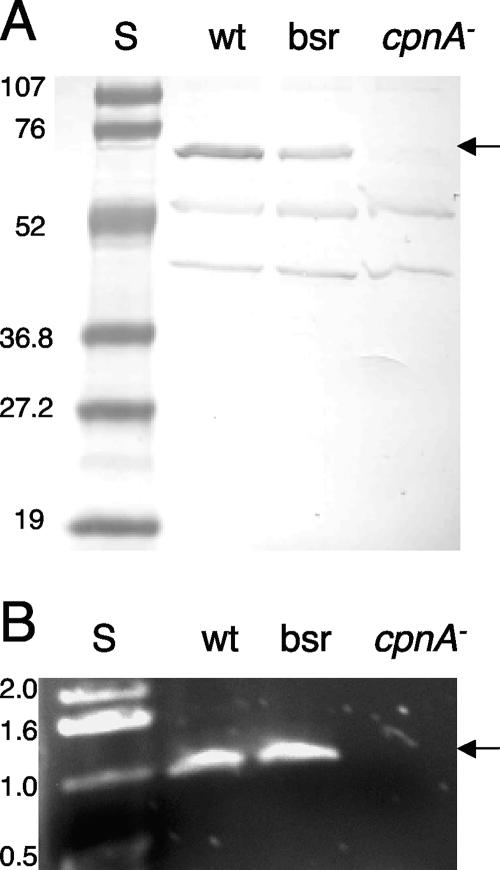
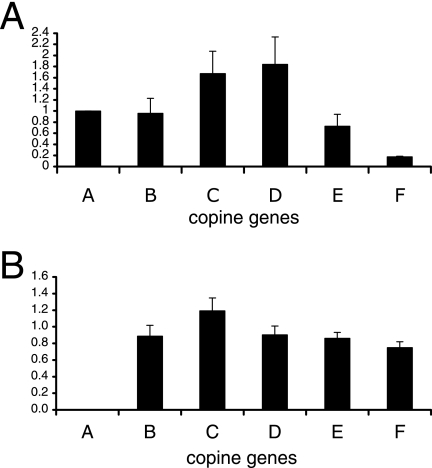
To investigate the function of CpnA in Dictyostelium, we created a cpnA− null mutant by homologous recombination and analyzed the phenotype. A DNA construct consisting of the blasticidin resistance gene (bsr) flanked by ∼1 kb of the upstream and downstream cpnA flanking sequences was electroporated into wild-type cells. The resultant blasticidin-resistant transformants were screened by Western blotting using antisera raised to a bacterially expressed fragment of CpnA. Sixteen out of the 20 blasticidin resistance clones screened did not express CpnA protein, as shown by Western blotting; the remaining four clones were blasticidin resistant yet still expressed CpnA. A Western blot with whole-cell samples of wild-type cells, one of the blasticidin-resistant clones expressing CpnA, and one of the cpnA− clones is shown in Fig. 1A. As seen in Fig. 1A, the CpnA antisera recognize two additional Dictyostelium proteins. These two proteins are found in all three cell strains, while CpnA is absent only from the cpnA− cells. To verify that the cpnA gene was removed from the genome in the cpnA− cells, we screened 3 out of the 16 cpnA− clones with PCR; we were not able to amplify the cpnA gene from these clones but were able to amplify the cpnA gene from wild-type cells and the blasticidin-resistant clones expressing CpnA (Fig. 1B). In addition, real-time RT-PCR studies of the same three cpnA− clones demonstrated that these clones do not produce detectable cpnA mRNA (Fig. 2B).
FIG. 1.
Analysis of cpnA− null mutants. (A) Whole-cell samples (5 × 106 cells per lane) from wild-type (wt) cells, nonhomologous recombination blasticidin-resistant (bsr) control cells, and cpnA− cells were analyzed by Western blotting with CpnA antiserum. Protein standards (S) are in the first lane, with molecular masses indicated in kDa. The arrow points to the CpnA protein band. (B) Wild-type cells, nonhomologous recombination blasticidin-resistant control cells, and cpnA− cells were lysed and analyzed by PCR using primers that amplify ∼1 kb of the cpnA gene. DNA standards are in the first lane, with lengths indicated in kb. The arrow points to the amplified cpnA PCR product. Both the CpnA protein and the cpnA gene are absent from cpnA− cells.
FIG. 2.
mRNA analysis using real-time RT-PCR of wild-type and cpnA− cells. Total RNA was isolated from wild-type and cpnA− cells grown on plates, and mRNA levels for each of the six copine genes (cpnA to cpnF are labeled A to F, respectively) were measured by real-time RT-PCR using a relative quantification protocol. (A) For wild-type cells, gene expression levels for cpnA-cpnF were normalized to the endogenous control gene, rnlA, and calibrated to the expression level of the cpnA gene. (B) For both wild-type and cpnA− cells, gene expression levels for cpnA-cpnF were normalized to the endogenous control gene, rnlA. The data are then expressed in terms of the expression level of each gene in cpnA− cells relative to the expression level of the same gene in wild-type cells. Data from four experiments were averaged and graphed, with error bars representing the standard error.
In our previous study, we identified six copine genes in the Dictyostelium genome (10). To measure the mRNA levels for each of the six copine genes identified in Dictyostelium, we used a relative quantification real-time RT-PCR protocol in which expression levels were normalized to an endogenous control gene, rnlA, which codes for a mitochondrial large ribosomal subunit rRNA. For wild-type vegetative cells growing on plates, mRNA levels of cpnA-cpnE were fairly similar, with levels of cpnC and cpnD typically 0.5- to 1.0-fold higher than that of cpnA. In contrast, the mRNA levels of cpnF were on average 5.6-fold lower than cpnA mRNA expression (Fig. 2A). When expression levels of each of the copine genes in cpnA− cells were compared to expression levels of the each of the copine genes in wild-type cells, cpnA expression was not detectable in cpnA− cells, and there was little difference in expression levels for cpnB-cpnF (Fig. 2B). This indicates that when growing on plates in nutrient-rich media, cpnA− cells do not compensate for the lack of cpnA by increasing expression of any of the other five copine genes.
cpnA− cells exhibit normal growth rates, but some are multinucleated.
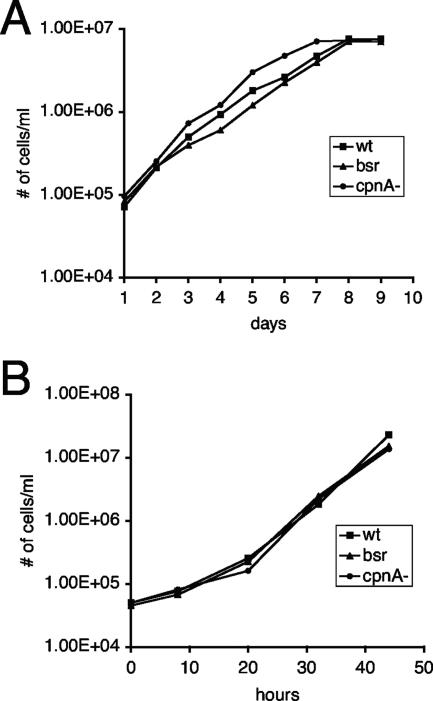
cpnA− cells typically appear to have a normal morphology. In addition, cpnA− cells are able to grow in suspension in axenic culture and feed on bacteria at rates similar to those seen for wild-type cells (Fig. 3A and B). However, some of the cpnA− cells appeared to be much larger than normal, which suggested that these cells may have a slight defect in cytokinesis. Mutant cells lacking myosin heavy chain II are not able to undergo cytokinesis when placed in suspension culture and become very large and multinucleated. However, although cytokinesis is still impaired when these cells are grown on dishes, they are able to divide, presumably by the daughter cells crawling away from each other (11).
FIG. 3.
cpnA− cells exhibit normal growth rates. Wild-type cells (wt), nonhomologous recombination blasticidin-resistant control cells (bsr), and cpnA− cells were grown in suspension in either HL-5 medium (A) or a phosphate buffer supplemented with E. coli B/r (B). Cell densities were estimated at various time points using a hemocytometer. One representative growth assay is shown for each.
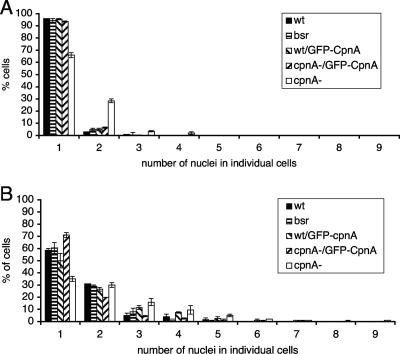
To determine if the larger-than-normal cpnA− cells were multinucleated, cells that had been growing in suspension and cells that had been growing on plates were fixed, the DNA was stained, and the nuclei were imaged using fluorescence microscopy. The abnormally large cells were indeed multinucleated. To quantify this defect, the numbers of nuclei in individual cpnA− cells growing on plates or in suspension were counted and compared to those for wild-type cells (Fig. 4A and B). cpnA− cells consistently had more nuclei than wild-type cells both when grown on plates (Fig. 4A) and when grown in suspension (Fig. 4B). Although a few cells had many nuclei (up to 20), most multinucleated cpnA− cells had 2 nuclei. Therefore, cpnA− cells appear to have only a slight cytokinesis defect that does not affect the overall growth rate. The observed cytokinesis defect was more pronounced in the newly created cpnA− cells and almost disappeared after several months of passage on plates. This cytokinesis defect was seen for three independent cpnA− cell clones tested. In addition, a GFP-tagged version of CpnA was expressed under the actin15 promoter in one of the cpnA− clones. The expression of GFP-CpnA completely rescued this phenotypic defect in cpnA− cells. Wild-type and cpnA− cells expressing GFP-CpnA had numbers of nuclei similar to those for wild-type cells (Fig. 4A and B).
FIG. 4.
cpnA− cells exhibit a slight cytokinesis defect. Wild-type cells (wt), nonhomologous recombination blasticidin-resistant control cells (bsr), wild-type cells expressing GFP-CpnA (wt/GFP-CpnA), cpnA− cells expressing GFP-CpnA (cpnA-/GFP-CpnA) and cpnA− cells (cpnA-) were grown in HL-5 on plates (A) or in suspension (B) for 6 days. Cell samples from each were fixed and stained with DAPI, and the numbers of nuclei in individual cells were counted using fluorescence microscopy. The percentages of cells containing a particular number of nuclei were calculated for two independent experiments and averaged. The error bars represent standard error.
Developmental expression patterns of the six copine genes.
Although Dictyostelium cells can live independently as single-celled amoebae, they undergo cell differentiation and morphogenesis to form multicellular structures when placed in starvation conditions. Dictyostelium cells placed in starvation buffer on filters at a particular density will characteristically complete their development in a 24-hour period. Upon starvation, cells undergo chemotaxis in response to periodic waves of secreted cyclic AMP and aggregate into hemispherical mounds at about 10 h after the beginning of starvation. Next, a tip is formed on the mound that elongates to give rise to a finger-shaped structure at 14 to 16 h. During the final stage of development, called culmination and beginning at 19 to 20 h, a dramatic change in morphology occurs, wherein cells terminally differentiate into spore or stalk cells to form fruiting bodies. Associated with differentiation and morphological changes during development is a regulated program of gene expression brought on by a variety of secreted factors (12, 23).
The mRNA levels for each of the six copine genes during Dictyostelium development were measured using a relative quantification real-time RT-PCR protocol. Because no single gene is expected to be expressed at the very same level throughout development, we used two different genes as our endogenous control genes: rnlA and cinD. rnlA, also known as IG7 and rrn, codes for a mitochondrial large subunit rRNA, while cinD, also known as H7, codes for a DNA binding protein (2). Both rnlA and cinD are considered to be constitutively expressed throughout development and have been used extensively as internal controls for nonquantitative RT-PCR analysis (39). Consistent with previous studies, the endogenous control genes were observed to have similar transcription levels, relative to each other, throughout development. However, in one of the two sets of RNA samples quantified, the rnlA mRNA levels were lower at the 24-h time point. Because of this result, there was an increase in the cinD expression level relative to that seen for rnlA and a larger standard error at the 24-h time point (Fig. 5H).
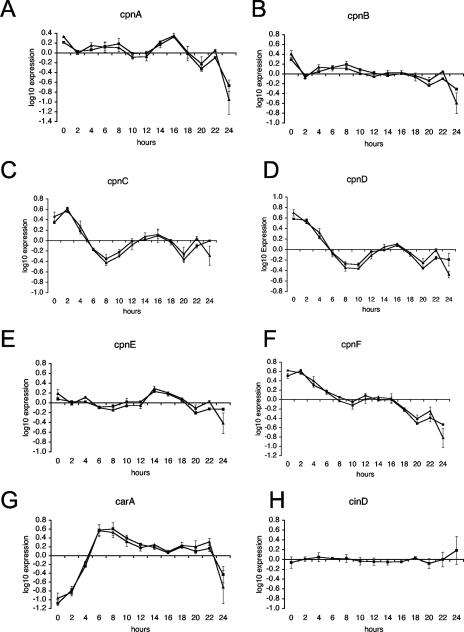
FIG. 5.
Developmental expression patterns of copine genes in Dictyostelium. Wild-type Dictyostelium cells were developed on filters, and cell samples were harvested every 2 h during the 24-h development. Total RNA was isolated from each cell sample, and mRNA levels were measured by real-time RT-PCR using a relative quantification protocol. Gene expression levels for cpnA to cpnF (panels A to F, respectively) and for the carA gene (G) were normalized to those of two endogenous control genes, rnlA (▪) and cinD (▴), and calibrated to an average expression level for each gene. Expression levels were then log10 transformed. Data from two experiments were averaged and graphed, with error bars representing the standard error. (H) Gene expression levels of the endogenous control gene cinD relative to that of the other endogenous control gene, rnlA.
In addition to the expression levels of the copine genes, we measured those of the carA gene throughout development as a positive control. carA, or cAR1, codes for a major cyclic AMP receptor that has previously been shown to be developmentally regulated. carA mRNA is undetectable in vegetative cells by Northern blot analysis, and its expression is upregulated early in development as cells are aggregating into mound structures. carA mRNA levels remain elevated through aggregation before steadily decreasing (37). Our observations of the expression pattern of carA were consistent with this pattern (Fig. 5G).
Our real-time RT-PCR data indicate that all six copine genes are expressed in vegetative cells and at various levels throughout development (Fig. 5A to F). All of the copine genes are upregulated at one or two developmental transition points, except cpnF, which shows decreasing expression throughout development. The cpnA gene was observed to have two peaks, a modest peak at the 8-hour time point and a threefold peak between the 12- and 16-hour time points; each of these peaks corresponds to a major transition during development. The 8-hour time point occurs just prior to mound formation, when cells transition from the unicellular to the multicellular stage. The 16-hour time point corresponds to the finger stage, when many of the prespore and prestalk genes are being activated (Fig. 5A). Three other copine genes, cpnC, cpnD, and cpnE, also exhibited an increase in mRNA expression that peaked at ∼16 h. All six copine genes were observed to have an approximately threefold decrease in expression from the 16-hour time point to the 20-hour time point, followed by a slight increase at the 22-hour time point before dropping at the 24-hour time point. Although all six copine genes of Dictyostelium are expressed at relatively high levels in vegetative cells (0 h), this study suggests that copine genes may be important regulators in Dictyostelium development.
cpnA− cells are arrested or delayed in the finger stage of development prior to culmination.
To determine if cpnA− cells are able to execute a normal developmental program, wild-type and cpnA− cells were allowed to develop on filters and bacterial lawns. Three independent cpnA− clones were developed on filters, and all three exhibited the defects described below. cpnA− cells appeared to begin development in a normal manner; the cells streamed and aggregated into small mounds with timing similar to that seen for wild-type cells. However, the cpnA− mounds appeared to be different than wild-type mounds in that we did not observe the characteristic C-shaped mounds due to the spiraling movement of cells within the mound (Fig. 6A and B). In addition, some of the mounds formed by cpnA− cells were irregularly shaped (Fig. 6B). Mounds formed by cpnA− cells proceeded to the finger stage in a normal time frame; however, they became delayed or arrested at the finger stage (Fig. 6C and D). Eventually, after another 24 h, some of the fingers developed into fruiting bodies, but most were smaller than wild-type fruiting bodies (Fig. 6E and F). The expression of GFP-CpnA under the actin15 promoter in cpnA− cells did not rescue the developmental defect; these cells were also delayed or arrested in the finger stage. This perhaps is not surprising, given that normal transcriptional regulation of cpnA is most likely very important to its proper function. Indeed, cpnA expression is upregulated at 16 h during development (Fig. 5A), while actin15 is downregulated at this stage (24).
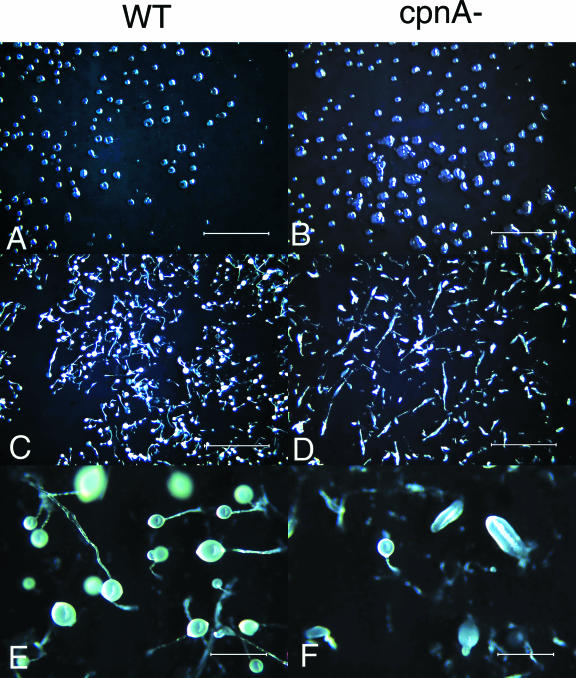
FIG. 6.
On filters, cpnA− cells are arrested or delayed in the finger stage of development. Wild-type (WT) and cpnA− (cpnA-) cells (5 × 107) were plated on filters in starvation buffer and allowed to develop. Images were taken with a dissecting microscope at various time points after plating. (A and B) 12 h; magnification, ×7; bar = 4 mm. (C and D) 24 h; magnification, ×7; bar = 4 mm. (E and F) 48 h; magnification, ×40; bar = 0.6 mm.
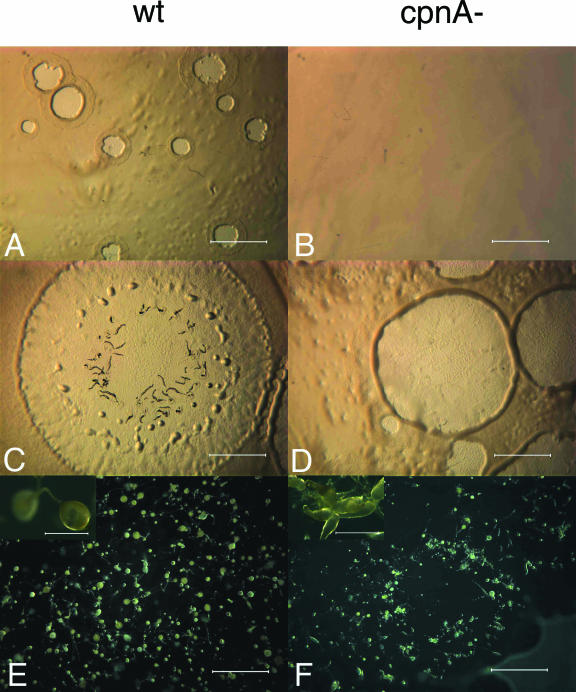
Cells mixed with bacteria and spread on agar plates will feed on the bacteria and divide to produce clear plaques within a bacterial lawn. As the cells in the center of the plaque starve, the cells will develop, resulting in plaques made up of cells at various stages of development. Six independent cpnA− clones were developed on bacterial lawns, and all six exhibited the defects described below. On bacterial lawns, cpnA− cells were delayed in plaque formation, with plaques appearing ∼24 h later than what was observed for wild-type cells (Fig. 7A and B). In addition, cpnA− cells made multifingered structures that accumulated at plaque edges, while wild-type cells formed normal single-fingered structures that filled the entire plate (Fig. 7E and F). As observed for cells developed on filters, cpnA− cells developed on bacterial lawns were delayed or arrested at the finger stage and made smaller-than-normal fruiting bodies (Fig. 7E and F). Our observations of the cpnA− mutant suggest that cpnA is necessary for the proper formation of mounds and fruiting bodies, and it is at these stages where there is an upregulation of cpnA gene expression during development.
FIG. 7.
On bacterial lawns, cpnA− (cpnA-) cells are delayed in plaque formation, make multifingered structures, and are arrested or delayed in the finger stage of development. Wild-type (wt) and cpnA− cells were mixed with E. coli B/r and plated on agar plates. Images were taken with a dissecting microscope at various time points after plating. (A and B) Day 3; magnification, ×7; bar = 4 mm. (C and D) Day 5; magnification, ×7; bar = 4 mm. (E and F) Day 7; magnification, ×7; bar = 4 mm. Insets in panels E and F are images taken from the same plates as in panels E and F; magnification, ×40; bar = 0.6 mm.
cpnA− cells contain unusually large contractile vacuoles when placed in water.
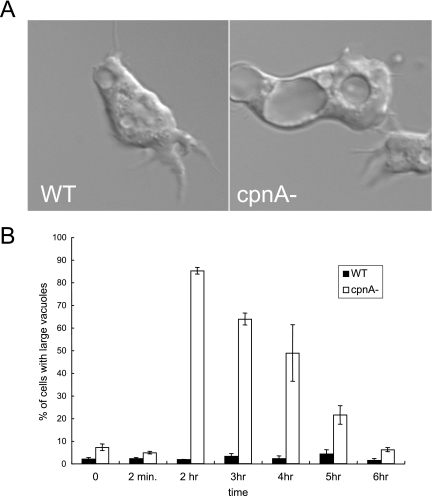
Because we previously found that GFP-CpnA associates with contractile vacuoles (10), an organelle involved in osmoregulation, we carried out experiments to determine whether cpnA− cells are able to behave normally in hypo-osmotic conditions. Wild-type and cpnA− cells were placed in water and observed using DIC microscopy. Within 30 min, cpnA− cells contained several vacuoles that were unusually large in comparison to those seen for wild-type cells (Fig. 8A; also see Movies S1 and S2 in the supplemental material). We identified these vacuoles as contractile vacuoles by immunofluorescence labeling with an antibody to calmodulin (20) as previously described (10) (data not shown). Time-lapse imaging of cpnA− cells placed in water revealed that cells are able to expel water from some of the smaller contractile vacuoles found within the cell; however, the time of discharge of the vacuoles appeared to be prolonged (see Movies S1 and S2 in the supplemental material). To quantify the large-vacuole problem, cpnA− and wild-type cells were placed in water, and the percentages of cells that contained at least one unusually large contractile vacuole were determined. Large vacuoles were arbitrarily designated as being larger than one-fourth of the area of the cell. Most of the cpnA− cells contained these unusually large vacuoles after 30 min to 1 h in water, while very few of the wild-type cells did. However, after 5 to 6 h in water, the cpnA− cells no longer had large vacuoles and looked similar to wild-type cells (Fig. 8B). Three independent cpnA− clones were observed in water, and they all exhibited the large-vacuole phenotype. Expression of GFP-CpnA under the actin15 promoter in the cpnA− cells was not able to rescue this defect.
FIG. 8.
cpnA− cells have unusually large contractile vacuoles when placed in water. (A) Wild-type (WT) and cpnA− (cpnA-) cells in HL-5 were placed in glass-bottom dishes and allowed to settle. The HL-5 was replaced with water, and after 2 h, the cells were imaged using DIC microscopy. (B) Wild-type and cpnA− clones in HL-5 were placed in glass-bottom dishes and allowed to settle. The HL-5 was replaced with water and the numbers of cells with vacuoles larger than one-fourth of the area of the cell were counted at various time points using DIC microscopy. The percentages of cells containing large vacuoles from three independent experiments were calculated and then averaged. The error bars represent standard error.
To determine if the defect in contractile vacuole function observed for cpnA− cells affects cell viability in water, cell viability assays with cpnA− and wild-type cells were carried out. We found that cpnA− cells had no decrease in cell viability in water from what was observed for wild-type cells (data not shown). This was not surprising, given that we had not observed excessive swelling or bursting of cpnA− cells when they were placed in water. cpnA− cells make unusually large contractile vacuoles soon after being placed in water but are able to recover within 5 to 6 h, suggesting that the cells may compensate for the loss of the cpnA gene by upregulating other genes. Therefore, we used real-time RT-PCR to determine whether copine genes are upregulated in wild-type cells and cpnA− cells in response to hypo-osmotic stress. However, we did not find that any of the copine genes were upregulated in response to hypo-osmotic stress in the course of several experimental trials.
cpnA− cells do not exhibit defects in rates of endocytosis, phagocytosis, and exocytosis.
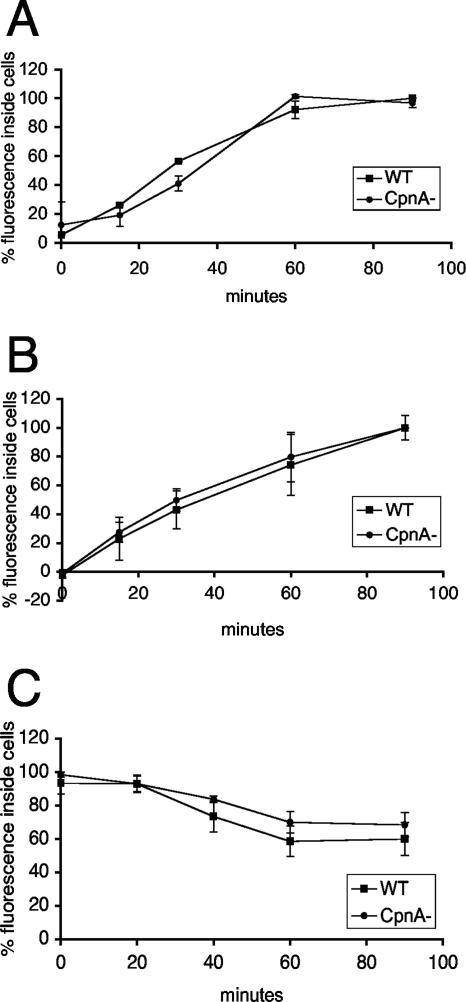
In our previous study, GFP-CpnA was found to associate with organelles of the endolysosomal pathway and phagosomes, suggesting that CpnA may have a role in endocytosis and/or phagocytosis (10). To assay the efficiency of endocytosis, the rate of uptake of FITC-conjugated dextran was measured by quantifying the amount of fluorescence inside cells at various time points after incubation with the FITC-dextran (Fig. 9A). To assay the efficiency of phagocytosis, the rate of uptake of fluorescently labeled beads of 1 μm in diameter was measured by again quantifying the amount of fluorescence inside cells at various time points after incubation with beads (Fig. 9B). To measure the rate of bead exocytosis, cells were first incubated with fluorescent beads for 1 hour and then washed and resuspended in buffer containing no beads; subsequently, the amount of fluorescence remaining inside the cells at various time points was quantified (Fig. 9C). For all three assays, the data were normalized by the protein content of each sample and expressed as percentages of fluorescence, with the highest fluorescence reading assigned the 100% value. Our results indicate that cpnA− cells do not exhibit any defects compared to wild-type cells in the rates of endocytosis and phagocytosis. Furthermore, cpnA− cells are able to exocytose beads taken up by phagocytosis at rates similar to those for wild-type cells.
FIG. 9.
cpnA− cells exhibit normal rates of endocytosis, phagocytosis, and exocytosis. Wild-type (WT) and cpnA− (CpnA-) cells in suspension were incubated with FITC-dextran (A) or 1.0-μm fluorescent beads (B and C). At various time points, cell samples were taken. The fluorescence intensity was measured and the protein concentration determined for each cell sample. The data were first calculated in terms of μg of FITC-dextran/μl fluorescent beads per mg of protein and then normalized, with the highest reading assigned the value of 100%. For the exocytosis assays (C), cells were first incubated with the beads for 1 hour and then washed, and then cell samples were taken. Data from three experimental trials were averaged, and standard errors were calculated.
DISCUSSION
Copines are calcium-dependent membrane binding proteins with hypothesized roles in signal transduction and membrane trafficking. Several studies provide evidence for copines functioning in a variety of signaling pathways that regulate growth, development, cell death, and defense (8, 19, 21, 22, 44, 47). Here, using the model organism Dictyostelium discoideum, we provide the first functional evidence for a copine having a role in organelle function and membrane trafficking. The Dictyostelium genome has six distinct copine genes, and Dictyostelium cells lacking one of these genes, the cpnA gene, exhibit a defect in contractile vacuole function. The contractile vacuole system, which functions in osmoregulation, is made up of membrane-bound tubules and bladders that connect with the plasma membrane to expel water. In addition, some studies indicate that the contractile vacuole system plays a role in calcium homeostasis and sequestration (28, 30). When Dictyostelium cpnA− cells are placed in water, they develop unusually large contractile vacuoles, indicating that CpnA has a role in regulating the volume of contractile vacuoles and/or the exocytosis of contractile vacuoles necessary for water expulsion.
cpnA− cells also exhibit other phenotypic defects. Many cpnA− cells contain more than one nucleus, suggesting inefficient cytokinesis. In addition, cpnA− cells become arrested or delayed in the final stages of development. Both of these phenotypic characteristics of the null mutant could also be attributed to defects in membrane trafficking. Studies with several cell types have shown that membrane trafficking at the cleavage furrow is necessary for cytokinesis (for reviews, see references 1, 29, and 35). Moreover, differentiation into spore cells during Dictyostelium development requires vesicle exocytosis (41). Dictyostelium cells lacking clathrin heavy chain, a well-characterized protein involved in vesicle budding, exhibit defects in endocytosis but also exhibit defects in cytokinesis, contractile vacuole function, and development (33, 34, 36). Alternatively, CpnA may have distinct roles in each of the three processes.
Several proteins have been shown to localize to or associate with contractile vacuoles in Dictyostelium; these include a Ca2+ ATPase (30), the vacuolar H+ ATPase (18), calmodulin (48), two Rab proteins (5, 17), drainin (3), dajumin (14), Rh50 (4), and LvsA (15). Mutant studies have shown that drainin (3), Rab11 (17), RabD (6), and LvsA (15) are each necessary for proper function of the contractile vacuole. Moreover, similar to the cpnA null mutant, drainin null mutant cells display large contractile vacuoles (3), as do cells expressing a dominant negative version of Rab11 (17). In addition and also similar to cpnA− cells, lvsA− cells exhibit defects in both cytokinesis and contractile vacuole function (15, 25). A detailed microscopic study of cytokinesis in Dictyostelium by Fukui and Inoué (13) revealed that the constriction of the cleavage furrow is accompanied by an efflux of water, as evidenced by highly active contractile vacuole formation during cytokinesis. This study suggests a function for the contractile vacuole system in cytokinesis and may explain the phenotypic defects in the cpnA− mutant and in lvsA− mutants.
It is also interesting to note that while Rab11 is associated with contractile vacuoles in Dictyostelium, the mammalian Rab11 is associated with endosomes, functions in endosome recycling, and has also recently been shown to be involved in the trafficking of endosomal vesicles to the cleavage furrow (46). Furthermore, when human embryonic kidney HEK293, human bladder carcinoma T24, and rat pheochromocytoma PC12 cells expressing a GFP-tagged human copine I are stimulated with carbachol or a calcium ionophore, the GFP-tagged copine is rapidly translocated from the cytosol to small peripherally located organelles, which are perhaps recycling endosomes (J. L. Tomsig and C. E. Creutz, unpublished data).
The overexpression of a N-terminally GFP-tagged CpnA in cpnA− cells is able to fully rescue the cytokinesis defect but not the contractile vacuole defect. Contrary to the idea that CpnA's role in cytokinesis may be secondary to its role in contractile vacuole function, as speculated above, the partial rescue of the phenotypic defects by the overexpression of GFP-CpnA suggests that CpnA has different roles in the two processes. While GFP-CpnA expression in cpnA− cells is able to fully rescue the cytokinesis defect, the overexpression or the GFP tag may interfere with its ability to rescue the contractile vacuole defect. Although we have previously shown that GFP-CpnA is able to bind membranes in a calcium-dependent manner (10), the GFP tag may alter the calcium sensitivity of CpnA's membrane binding activity. In addition, the C2 domains of synaptotagmin I have been shown to bind to several different proteins (42); therefore, it is possible that the GFP at the N terminus interferes with the binding of proteins to the C2 domains of CpnA.
Copine mutant studies with Arabidopsis indicate that copines function to promote growth and development (47). Similarly, our study suggests that copine genes may be important regulators in Dictyostelium development. cpnA− cells are able to aggregate into mounds with normal timing; however, the mounds are irregularly shaped. Cells continue to develop but become delayed or arrested in the finger stage, prior to culmination. These developmental defects are observed at transitional stages that correspond to modest increases in expression of the cpnA gene in wild-type cells (Fig. 7A).
We also determined the developmental expression patterns of the other five copine genes in Dictyostelium. Each of the six copine genes exhibited a distinct pattern of expression throughout development, suggesting that each of the genes carries out a distinct function in development. All of the copine genes except cpnF show an upregulation of expression at one or two developmental transitions. All six copine genes of Dictyostelium (cpnA-cpnF) are expressed at relatively high levels in vegetative cells (0 h). cpnC is up-regulated at the 2-hour time point and may be involved in early aggregation, while cpnA and cpnB exhibit a small increase in expression around the 8-hour time point, suggesting these genes may have a role in mound formation. cpnA, cpnC, cpnD, and cpnE exhibit an increase in mRNA levels that peak at 14 to 16 h, suggesting that these genes may be involved in the differentiation of cells into prespore or prestalk cells. The study of Yang et al. (47) on copine mutants of Arabidopsis suggests that copines are involved not only in promoting cell growth and development but also in repressing cell death. Perhaps some of the copine genes are up-regulated in prespore cells but not prestalk cells, leading to the ultimate survival of spore cells and the death of stalk cells during the final stages of culmination. Determining if the various copine genes are preferentially expressed in either prespore or prestalk cells will offer further insight into the function of copines genes during Dictyostelium development.
If the general function of copines is to bind to specific target proteins and carry them to particular membranes, then CpnA may bind to a protein necessary for targeted exocytosis and bring that protein to the contractile vacuole membrane during hypo-osmotic stress, to vesicles targeted for the plasma membrane at the cleavage furrow during cytokinesis, and to vesicles targeted to the plasma membrane during spore cell differentiation. Alternatively, CpnA may have roles in cytokinesis, contractile vacuole function, and development, because it is able to bind to several different target proteins. Proteomic analysis of the midbody of mammalian cells undergoing cytokinesis identified a copine protein as a component of the midbody (40). We have not observed GFP-CpnA to localize to the cleavage furrow or midbody in Dictyostelium, but such localization may be very transient, given that our previous studies with GFP-CpnA indicated that CpnA localization to contractile vacuoles in live cells lasts for only a few seconds (10). If the function of CpnA is to facilitate the targeting of a particular protein to the contractile vacuole during hypo-osmotic stress, this may explain why cpnA− cells display a contractile vacuole defect for only a few hours after being placed in water. Perhaps without the help of CpnA, it takes much longer for the target protein to accumulate on the contractile vacuole membrane. In addition, the mild cytokinesis defect and the transient contractile defect observed for the cpnA− cells may be due to the fact that the Dictyostelium copines have some overlapping functions and that the creation of multiple copine knockout strains would produce more-severe phenotypes. Copine mutant studies with Arabidopsis have shown that double and triple copine gene deletion mutants exhibit phenotypes more severe than those of the single gene deletion mutants (47). To fully understand the function of copines in Dictyostelium, it is critical to identify their target proteins, and we are currently working to identify the binding partners of CpnA.
Supplementary Material
Acknowledgments
We thank Abigail Proffer for PCR cloning of the 5′ flanking sequence of the cpnA gene, Theresa J. O'Halloran for sending us the pBSIIbsr plasmid, and Jerry Calvin for microscopy technical assistance.
This study was funded by a National Science Foundation (grant 0110555), a Merck/AAAS Undergraduate Science Research Program grant, the Undergraduate Research Summer Institute at Vassar College, and a grant from the Camille and Henry Dreyfus Foundation Special Grant Program in the Chemical Sciences.
Footnotes
Published ahead of print on 26 January 2007.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Albertson, R., B. Riggs, and W. Sullivan. 2005. Membrane traffic: a driving force in cytokinesis. Trends Cell Biol. 15:92-101. [DOI] [PubMed] [Google Scholar]
- 2.Bakke, A. C., and J. Bonner. 1979. Purification and the histones of Dictyostelium discoideum chromatin. Biochemistry 18:4556-4562. [DOI] [PubMed] [Google Scholar]
- 3.Becker, M., M. Matzner, and G. Gerisch. 1999. Drainin required for membrane fusion of the contractile vacuole in Dictyostelium is the prototype of a protein family also represented in man. EMBO J. 18:3305-3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benghezal, M., D. Gotthardt, S. Cornillon, and P. Cosson. 2001. Localization of the Rh50-like protein to the contractile vacuole in Dictyostelium. Immunogenetics 52:284-288. [DOI] [PubMed] [Google Scholar]
- 5.Bush, J., K. Nolta, J. Rodriguez-Paris, N. Kaufmann, T. O'Halloran, T. Ruscetti, L. Temesvari, T. Steck, and J. Cardelli. 1994. A Rab4-like GTPase in Dictyostelium discoideum colocalizes with V-H(+)-ATPases in reticular membranes of the contractile vacuole complex and in lysosomes. J. Cell Sci. 107:2801-2812. [DOI] [PubMed] [Google Scholar]
- 6.Bush, J., L. Temesvari, J. Rodriguez-Paris, G. Buczynski, and J. Cardelli. 1996. A role for a Rab4-like GTPase in endocytosis and in regulation of contractile vacuole structure and function in Dictyostelium discoideum. Mol. Biol. Cell 7:1623-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chisholm, R. L., P. Gaudet, E. M. Just, K. E. Pilcher, P. Fey, S. N. Merchant, and W. A. Kibbe. 2006. dictyBase, the model organism database for Dictyostelium discoideum. Nucleic Acids Res. 34:D423-D427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Church, D. L., and E. J. Lambie. 2003. The promotion of gonadal cell divisions by the Caenorhabditis elegans TRPM cation channel GON-2 is antagonized by GEM-4 copine. Genetics 165:563-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Creutz, C. E., J. L. Tomsig, S. L. Snyder, M. C. Gautier, F. Skouri, J. Beisson, and J. Cohen. 1998. The copines, a novel class of C2 domain-containing, calcium-dependent, phospholipid-binding proteins conserved from Paramecium to humans. J. Biol. Chem. 273:1393-1402. [DOI] [PubMed] [Google Scholar]
- 10.Damer, C. K., M. Bayeva, E. S. Hahn, J. Rivera, and C. I. Socec. 2005. Copine A, a calcium-dependent membrane-binding protein, transiently localizes to the plasma membrane and intracellular vacuoles in Dictyostelium. BMC Cell Biol. 6:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Lozanne, A., and J. A. Spudich. 1987. Disruption of the Dictyostelium myosin heavy chain by homologous recombination. Science 236:1086-1091. [DOI] [PubMed] [Google Scholar]
- 12.Escalante, R., and J. J. Vicente. 2000. Dictyostelium discoideum: a model system for differentiation and patterning. Int. J. Dev. Biol. 44:819-835. [PubMed] [Google Scholar]
- 13.Fukui, Y., and S. Inoué. 1991. Cell division in Dictyostelium with special emphasis on actomyosin organization in cytokinesis. Cell Motil. Cytoskelet. 18:41-54. [DOI] [PubMed] [Google Scholar]
- 14.Gabriel, D., U. Hacker, J. Köhler, A. Müller-Taubenberger, J. M. Schwartz, M. Westphal, and G. Gerisch. 1999. The contractile vacuole network of Dictyostelium as a distinct organelle: its dynamics visualized by a GFP marker protein. J. Cell Sci. 112:3995-4005. [DOI] [PubMed] [Google Scholar]
- 15.Gerald, N. J., M. Siano, and A. De Lozanne. 2002. The Dictyostelium LvsA protein is localized on the contractile vacuole and is required for osmoregulation. Traffic 3:50-60. [DOI] [PubMed] [Google Scholar]
- 16.Gottschalk, A., R. B. Almedom, T. Schedletzky, S. D. Anderson, J. R. Yates III, and W. R. Schafer. 2005. Identification and characterization of novel nicotinic receptor-associated proteins in Caenorhabditis elegans. EMBO J. 24:2566-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris, E., K. Yoshida, J. Cardelli, and J. Bush. 2001. Rab11-like GTPase associates with and regulates the structure and function of the contractile vacuole system in Dictyostelium. J. Cell Sci. 114:3035-3045. [DOI] [PubMed] [Google Scholar]
- 18.Heuser, J., Q. Zhu, and M. Clarke. 1993. Proton pumps populate the contractile vacuoles of Dictyostelium amoebae. J. Cell Biol. 121:1311-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hua, J., P. Grisafi, S. H. Cheng, and G. R. Fink. 2001. Plant growth homeostasis is controlled by the Arabidopsis BON1 and BAP1 genes. Genes Dev. 15:2263-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hulen, D., B. Baron, J. Salisbury, and M. Clarke. 1991. Production and specificity of monoclonal antibodies against calmodulin from Dictyostelium discoideum Cell Motil. Cytoskelet. 18:113-122. [DOI] [PubMed] [Google Scholar]
- 21.Jambunathan, N., and T. W. McNellis. 2003. Regulation of Arabidopsis COPINE 1 gene expression in response to pathogens and abiotic stimuli. Plant Physiol. 132:1370-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jambunathan, N., J. M. Siani, and T. W. McNellis. 2001. A humidity-sensitive Arabidopsis copine mutant exhibits precocious cell death and increased disease resistance. Plant Cell 13:2225-2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kessin, R. H. 2001. Dictyostelium: evolution, cell biology, and the development of multicellularity. Cambridge University Press, Cambridge, United Kingdom.
- 24.Knecht, D. A., S. M. Cohen, W. F. Loomis, and H. F. Lodish. 1986. Developmental regulation of Dictyostelium discoideum actin gene fusions carried on low-copy and high-copy transformation vectors. Mol. Cell. Biol. 6:3973-3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwak, E., N. Gerald, D. A. Larochelle, K. K. Vithalani, M. L. Niswonger, M. Maready, and A. De Lozanne. 1999. LvsA, a protein related to the mouse beige protein, is required for cytokinesis in Dictyostelium. Mol. Biol. Cell 10:4429-4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu, J., N. Jambunathan, and T. W. McNellis. 2005. Transgenic expression of the von Willebrand A domain of the BONZAI 1/COPINE 1 protein triggers a lesion-mimic phenotype in Arabidopsis. Planta 221:85-94. [DOI] [PubMed] [Google Scholar]
- 27.Maitra, R., D. N. Grigoryev, T. K. Bera, I. H. Pastan, and B. Lee. 2003. Cloning, molecular characterization, and expression analysis of Copine 8. Biochem. Biophys. Res. Commun. 303:842-847. [DOI] [PubMed] [Google Scholar]
- 28.Malchow, D., D. F. Lusche, C. Schlatterer, A. De Lozanne, and A. Müller-Taubenberger. 2006. The contractile vacuole in Ca2+-regulation in Dictyostelium: its essential function for cAMP-induced Ca2+-influx. BMC Dev. Biol. 6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matheson, J., X. Yu, A. B. Fielding, and G. W. Gould. 2005. Membrane traffic in cytokinesis. Biochem. Soc. Trans. 33:1290-1294. [DOI] [PubMed] [Google Scholar]
- 30.Moniakis, J., M. B. Coukell, and A. Janiec. 1999. Involvement of the Ca2+-ATPase PAT1 and the contractile vacuole in calcium regulation in Dictyostelium discoideum. J. Cell Sci. 112:405-414. [DOI] [PubMed] [Google Scholar]
- 31.Nakayama, T., T. Yaoi, and G. Kuwajima. 1999. Localization and subcellular distribution of N-copine in mouse brain. J. Neurochem. 72:373-379. [DOI] [PubMed] [Google Scholar]
- 32.Nalefski, E. A., and J. J. Falke. 1996. The C2 domain calcium-binding motif: structural and functional diversity. Protein Sci. 5:2375-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niswonger, M. L., and T. J. O'Halloran. 1997. Clathrin heavy chain is required for spore cell but not stalk cell differentiation in Dictyostelium discoideum. Development 124:443-451. [DOI] [PubMed] [Google Scholar]
- 34.Niswonger, M. L., and T. J. O'Halloran. 1997. A novel role for clathrin in cytokinesis. Proc. Natl. Acad. Sci. USA 94:8575-8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Halloran, T. J. 2000. Membrane traffic and cytokinesis. Traffic 1:921-926. [PubMed] [Google Scholar]
- 36.O'Halloran, T. J., and R. G. Anderson. 1992. Clathrin heavy chain is required for pinocytosis, the presence of large vacuoles, and development in Dictyostelium. J. Cell Biol. 118:1371-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saxe, C. L., III, R. Johnson, P. N. Devreotes, and A. R. Kimmel. 1991. Multiple genes for cell surface cAMP receptors in Dictyostelium discoideum. Dev. Genet. 12:6-13. [DOI] [PubMed] [Google Scholar]
- 38.Shelden, E., and D. A. Knecht. 1995. Mutants lacking myosin II cannot resist forces generated during multicellular morphogenesis. J. Cell Sci. 108:1105-1115. [DOI] [PubMed] [Google Scholar]
- 39.Singleton, C. K., M. J. Zinda, B. Mykytka, and P. Yang. 1998. The histidine kinase dhkC regulates the choice between migrating slugs and terminal differentiation in Dictyostelium discoideum. Dev. Biol. 203:345-357. [DOI] [PubMed] [Google Scholar]
- 40.Skop, A. R., H. Liu, J. Yates III, B. J. Meyer, and R. Heald. 2004. Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science 305:61-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srinivasan, S., H. Alexander, and S. Alexander. 2000. Crossing the finish line of development: regulated secretion of Dictyostelium proteins. Trends Cell Biol. 10:215-219. [DOI] [PubMed] [Google Scholar]
- 42.Südhof, T. C., and J. Rizo. 1996. Synaptotagmins: C2-domain proteins that regulate membrane traffic. Neuron 17:379-388. [DOI] [PubMed] [Google Scholar]
- 43.Tomsig, J. L., S. L. Snyder, and C. E. Creutz. 2003. Identification of targets for calcium signaling through the copine family of proteins. Characterization of a coiled-coil copine-binding motif. J. Biol. Chem. 278:10048-10054. [DOI] [PubMed] [Google Scholar]
- 44.Tomsig, J. L., H. Sohma, and C. E. Creutz. 2004. Calcium-dependent regulation of tumour necrosis factor-alpha receptor signalling by copine. Biochem. J. 378:1089-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whittaker, C. A., and R. O. Hynes. 2002. Distribution and evolution of von Willebrand/integrin A domains: widely dispersed domains with roles in cell adhesion and elsewhere. Mol. Biol. Cell 13:3369-3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson, G. M., A. B. Fielding, G. C. Simon, X. Yu, P. D. Andrews, R. S. Hames, A. M. Frey, A. A. Peden, G. W. Gould, and R. Prekeris. 2005. The FIP3-Rab11 protein complex regulates recycling endosome targeting to the cleavage furrow during late cytokinesis. Mol. Biol. Cell 16:849-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang, S., H. Yang, P. Grisafi, S. Sanchatjate, G. R. Fink, Q. Sun, and J. Hua. 2006. The BON/CPN gene family represses cell death and promotes cell growth in Arabidopsis. Plant J. 45:166-179. [DOI] [PubMed] [Google Scholar]
- 48.Zhu, Q., and M. Clarke. 1992. Association of calmodulin and an unconventional myosin with the contractile vacuole complex of Dictyostelium discoideum. J. Cell Biol. 118:347-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.