Abstract
Blood culture bottles with antimicrobial removal systems are recommended for patients who develop fever while on antibiotics. This study compared the ability of Becton Dickinson (Sparks, MD) BACTEC PLUS bottles and bioMerieux (Durham, NC) BacT/Alert FA bottles to effectively remove vancomycin, cefoxitin, ceftriaxone, cefepime, piperacillin-tazobactam, ampicillin, oxacillin, gentamicin, and a combination of gentamicin/penicillin, thus allowing bacterial pathogens to grow. Each bottle was spiked with 10 ml of human blood, antibiotic, and strains of organisms susceptible to the antibiotic evaluated. The organisms used were type strains and clinical isolates of Staphylococcus aureus (methicillin susceptible and resistant), Streptococcus pneumoniae, a viridans streptococcus, Enterococcus faecalis, Enterococcus faecium, Streptococcus agalactiae, Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Testing was completed in triplicate, using 10 to 100 CFU/ml of organisms with various concentrations of each antibiotic. Two rounds of testing were completed per antibiotic/organism combination. Bottles were mixed and loaded onto their respective instruments as per the manufacturer's instructions. Antimicrobial removal was evaluated on the basis of time to detection of organism growth, for up to 5 days of incubation. Overall, the BacT/Alert FA system recovered 25.1% of strains from test bottles and 96.9% of strains from growth control bottles (no antibiotic added), and the BACTEC PLUS system recovered 95.1% of strains from test bottles and 100% of strains from growth control bottles. Both systems performed well in the detection of Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa in the presence of gentamicin. In the presence of ceftriaxone, neither system was able to recover Streptococcus pneumoniae. The ability to remove vancomycin and cefoxitin was also determined by measuring antibiotic levels remaining in bottles after 1 h of incubation. The results demonstrated remaining levels of 72 to 90% of vancomycin and 71 to 72% of cefoxitin in the BacT/Alert system. For the BACTEC system, remaining levels were 0 to 30% of vancomycin and 0% of cefoxitin. Under these simulated conditions, the BACTEC PLUS system was superior to the BacT/Alert FA system in recovering gram-positive and gram-negative bacterial pathogens in the presence of β-lactam antibiotics, gentamicin/penicillin, and vancomycin.
Approximately 250,000 bloodstream infections are reported each year in the United States (17). Timely identification of the responsible pathogen permits targeted broad-spectrum empirical therapy and, if required, institution of infection control precautions. Identification of the etiologic agent is particularly important in determining the type and duration of treatment in patients with endocarditis and in predicting the likely response to therapy (5). Mortality attributed to bacteremia ranges from 20% to 50% (11). It has been shown that mortality associated with bacteremia is affected by the antimicrobial therapy administered. In the study by Weinstein et al. (23), patients who received appropriate antimicrobial therapy during initial empirical therapy, after the blood culture was reported positive, and after susceptibility results became available had the lowest septicemia-associated mortality (23). Patients whose initial empirical therapy was not appropriate but changed after the blood culture was reported positive also had a low mortality rate. Outcomes were poor for those patients whose antibiotics were changed after receipt of susceptibility testing results or remained incorrect throughout the course of illness (23).
Among patients for whom blood cultures have been obtained, 28 to 63% are on antibiotic therapy at the time of blood draw (18, 23). This can negatively affect the recovery of the etiologic agent. Concerns about the inability to recover a pathogen due to antibiotic therapy are well documented in the literature. In 1945, Dowling and Hirsh (8) published an article about the use of penicillinase in cultures of body fluids obtained from patients undergoing treatment with penicillin. In 1963, a review article in the Journal of the American Medical Association demonstrated the unmasking of false-negative blood cultures in patients receiving new penicillins when penicillinase produced by Bacillus cereus was added to blood cultures (6). The practical utility of B. cereus penicillinase was demonstrated in limited patient studies, leading to the recommendation that 8,000 units of B. cereus penicillinase per milliliter of blood be added to bacterial cultures from treated patients. Subsequent to these early studies, blood culture manufacturers have devised methods for detection of bacterial pathogens in patients on antimicrobial therapy (4, 25). These include antibiotic-inactivating resins and media containing charcoal (3, 14, 22, 24). While there are abundant clinical studies which demonstrate enhanced recovery of common bacterial pathogens and yeasts in resin and charcoal-containing media (3, 9, 10, 12, 13, 15, 18, 22, 23, 24), few studies compare the ability of the materials in them to neutralize standard concentrations of antimicrobials (7, 14, 16, 19, 21, 25).
This study directly compared the ability of BACTEC PLUS bottles (BD Diagnostics, Sparks, MD) and BacT/Alert FA bottles (bioMerieux, Durham, NC) to inactivate cefoxitin, piperacillin-tazobactam, vancomycin, gentamicin, gentamicin with penicillin, ampicillin, and cefepime at therapeutic concentrations and to allow select bacterial pathogens to grow. Also analyzed were the reduction of cefoxitin in the growth medium over time and the reduction of vancomycin in the growth medium over time, using an agar well diffusion method (bioassay) and a quantitative immunoassay, respectively.
MATERIALS AND METHODS
Media.
The blood culture media used in the study were BACTEC PLUS Aerobic/F and BacT/Alert FA. The BACTEC PLUS system utilizes an antibiotic-binding resin bead technology for the removal of antibiotics. The BacT/Alert system contains Ecosorb, a substance that contains absorbent charcoal material and Fuller's earth for the removal of antibiotics. Multiple lots of each medium type were all used well within the expiration date for each blood culture system.
Antibiotics.
Antibiotics were diluted to yield final concentrations consistent with trough, mid, and peak therapeutic serum levels (1, 20). The antibiotics chosen for testing represented those most frequently used at the Johns Hopkins Hospital. Antibiotics were measured and prepared on each day of use. The final concentrations of the antibiotics were as follows: ampicillin, 3 μg/ml, 22 μg/ml, and 47 μg/ml; cefepime, 10 μg/ml, 87 μg/ml, and 164 μg/ml; cefoxitin, 10 μg/ml, 60 μg/ml, and 110 μg/ml; ceftriaxone, 94 μg/ml, 125 μg/ml, and 250 μg/ml; gentamicin, 1 μg/ml, 4 μg/ml, and 8 μg/ml; gentamicin/penicillin combination, 0.5/0.08 μg/ml, 1.75/10 μg/ml, and 3/20 μg/ml; oxacillin, 13 μg/ml, 57 μg/ml, and 230 μg/ml; piperacillin-tazobactam, 5/0.7 μg/ml, 100/10 μg/ml, and 240/24 μg/ml; vancomycin, 10 μg/ml, 25 μg/ml, and 50 μg/ml. Concentrations of each antibiotic were calculated based on maximum daily dosing for a 70-kg adult with normal renal function.
Organisms.
The organisms were serially diluted to achieve a final concentration of 10 to 100 CFU/ml. Colony counts were carried out to confirm concentrations. One diluted stock solution per organism was used for all testing per round. For cefoxitin and piperacillin-tazobactam, both rounds of testing included ATCC type strains of gram-positive and gram-negative pathogens. The following ATCC strains were used: methicillin-susceptible Staphylococcus aureus ATCC 25923, Streptococcus pneumoniae ATCC 49619, Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 33495, and Pseudomonas aeruginosa ATCC 27853.
For vancomycin, the initial round of testing included ATCC and three clinical strains of gram-positive pathogens. The clinical strains consisted of Enterococcus faecium, Enterococcus faecalis, and a viridans streptococcus isolate. The second round of testing used the following ATCC strains: methicillin-susceptible S. aureus ATCC 25923, methicillin-resistant S. aureus ATCC 43300, E. faecium ATCC 35667, E. faecalis ATCC 49533, Streptococcus oralis ATCC 10557, and S. pneumoniae ATCC 49619. For ampicillin, both rounds of testing were completed using E. faecalis ATCC 49533, S. agalactiae ATCC 12385, and S. pneumoniae ATCC 49619. With oxacillin, methicillin-susceptible S. aureus ATCC 25923 was used; with the gentamicin/penicillin combination, E. faecalis ATCC 49533 was tested; and with ceftriaxone, S. pneumoniae ATCC 49619 was the challenge isolate. For gentamicin and cefepime, E. coli ATCC 25922, K. pneumoniae ATCC 33495, and P. aeruginosa ATCC 27853 were tested. All strains used were susceptible to the antibiotics with which they were tested.
Bottle inoculation/incubation.
Bottles were inoculated with 10 ml of banked blood purchased from Tennessee Interstate Labs drawn not more than 5 days prior to use and stored at 4°C. After the addition of blood, antibiotics were added to the bottles. One set of each bottle type had no antibiotic added and served as a growth control. After the addition of blood and antibiotics, an inoculum of 10 to 100 CFU of organisms was added to all bottles. The bottles were inverted to mix. Each set of bottles—a growth control and the three concentrations of antibiotics—was tested in triplicate with each organism.
Immediately after inoculation, the bottles were loaded into the instruments for a 5-day incubation protocol. When machines flagged cultures as positive, the bottles were pulled and gram staining and subculturing were completed. If the gram stain was negative despite a positive signal, the bottle was reloaded for continued incubation.
This protocol was repeated in duplicate on 2 different days. Data are shown as cumulative results obtained from both days of testing.
Data analysis.
Data management was through an SQL Server 2000 database, with analyses performed using SAS version 9.1.3 (SAS Institute Inc., Cary, NC). The total values for recovery with BACTEC PLUS and BacT/Alert FA were compared using McNemar's chi-square test of differences with a continuity correction at an α value of 0.05.
Agar well diffusion method (bioassay) for cefoxitin.
The remaining amounts of cefoxitin in culture media were analyzed. This analysis was performed to determine whether the amounts of antibiotic remaining correlated with the ability of the media to support the growth of the organisms.
Bottles were inoculated with 10 ml of banked blood and various concentrations of cefoxitin. Two microliters was immediately removed and centrifuged at 1,400 rpm for 10 min. Bottles were loaded into instruments and incubated for 1 h, and then an additional 2 ml was removed and centrifuged as before. The antibiotic levels in all samples were analyzed by using the agar well diffusion method (bioassay). The bioassay was done according to the established standard protocol (2). S. aureus ATCC 29213 was used for testing. Organisms were grown on 5% sheep blood agar medium for 24 h and inoculated into 5 ml of Mueller-Hinton broth. The Mueller-Hinton broth tubes were incubated in a water bath at 37°C until the turbidity reached a 0.5 McFarland standard concentration. Subsequently, a 1:10 dilution of this S. aureus suspension was made. Four milliliters of each dilution was added to conical tubes of liquefied medium that were inverted to mix. The medium was then poured into 150-mm plates and allowed to harden. A 3-mm-diameter sterile metal tube was used to punch holes in the medium for testing. Each hole was then inoculated with 5 μl of cefoxitin (concentrations: 25, 50, 100, and 200 μg/ml), controls, and samples from bottles in triplicate. All plates were incubated overnight at 37°C. Mean zone sizes were calculated. Sample concentrations were calculated using a cefoxitin standard curve derived from wells containing known cefoxitin concentrations. The lower limit of the assay was 10 μg/ml; therefore, trough levels could not be absolutely determined.
Quantitative immunoassay for vancomycin.
Remaining amounts of vancomycin were analyzed. This analysis was performed to determine whether the amount of antibiotic remaining correlated with the ability of the medium to support the growth of the organisms.
Bottles were inoculated with 10 ml of banked blood and various concentrations of vancomycin. Two microliters was immediately removed and centrifuged at 1,400 rpm for 10 min. Bottles were loaded into instruments and incubated for 1 h, and then an additional 2 ml was removed and centrifuged as before. The vancomycin levels in all samples were analyzed using the EMIT 2000 vancomycin assay (Syva, Cupertino, CA). The assay is a homogeneous enzyme immunoassay technique used for the quantitative analysis of vancomycin in human serum or plasma.
RESULTS
The overall recovery of bacterial isolates from the BACTEC PLUS system was 95.1% (616/648), with 100% of the growth control strains and 93.4% of the challenge strains being recovered. The overall recovery of bacterial isolates from the BacT/Alert FA system was 43.1% (279/648), with 96.9% of the strains from the growth control bottles and 25.1% of the strains from the test bottles being recovered. The difference in the rates of recovery from the two media was statistically significant (P = 0.0001) (Table 1).
TABLE 1.
Overall growth of organisms from growth control and test bottles for the BACTEC PLUS and the BacT/Alert FA systems
| Organism recovery | No. of organisms recovered/total (%) with indicated system
|
|
|---|---|---|
| BACTEC PLUS | BacT/Alert FA | |
| Overall | 616/648 (95.1)a | 279/648 (43.1) |
| From growth control bottles | 162/162 (100) | 157/162 (96.9) |
| From test bottles | 454/486 (93.4) | 122/486 (25.1) |
Results are statistically significant (P = 0.0001).
Both systems performed well in the detection of pathogens in the presence of gentamicin. All isolates of E. coli, K. pneumoniae, and P. aeruginosa were recovered. The average times to detection (TTD) were 11.0 h for BACTEC PLUS and 13.0 h for BacT/Alert FA. In the presence of ceftriaxone, neither system was able to recover S. pneumoniae. Times to detection for the strains from the growth control bottles were 9.9 h for BACTEC PLUS and 15.3 h for BacT/Alert FA.
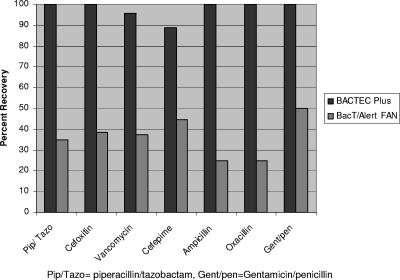
Various results were seen with the remaining antibiotics (Fig. 1 and Tables 2 and 3). For piperacillin-tazobactam, overall recovery for the BACTEC PLUS system and the BacT/Alert system was 100% (120/120) and 35.0% (42/120) of strains, respectively. BACTEC PLUS recovered 100% of strains from both the growth control and the test bottles at all levels of antibiotic. With the BacT/Alert system, 100% of strains from the control bottles were recovered, with 33%, 6.7%, and 0% of strains from the test bottles being recovered at trough levels, mid levels, and peak levels, respectively. The average times to detection were 12.4 h and 14.5 h for the BACTEC PLUS system and the BacT/Alert system, respectively.
FIG. 1.
Percent recovery of control and challenge organisms in BACTEC PLUS and BacT/Alert FA bottles containing antibiotics. Abbreviations: Pip/Tazo, piperacillin/tazobactam; Gent/pen, gentamicin/penicillin.
TABLE 2.
Percent recovery of various gram-negative pathogens for each system at each antimicrobial level
| Drug(s) | Concna | % Recovery of organism with indicated system
|
|||||
|---|---|---|---|---|---|---|---|
|
E. coli ATCC 25922
|
K. pneumoniae ATCC 49619
|
P. aeruginosa ATCC 27853
|
|||||
| BACTEC PLUS | BacT/Alert FA | BACTEC PLUS | BacT/Alert FA | BACTEC PLUS | BacT/Alert FA | ||
| Gentamicin | T | 100 | 100 | 100 | 100 | 100 | 100 |
| M | 100 | 100 | 100 | 100 | 100 | 100 | |
| P | 100 | 100 | 100 | 100 | 100 | 100 | |
| Cefepime | T | 100 | 50 | 100 | 50 | 100 | 100 |
| M | 66 | 0 | 100 | 0 | 100 | 33 | |
| P | 0 | 0 | 100 | 0 | 100 | 0 | |
| Cefoxitin | T | 100 | 100 | 100 | 17 | NTb | NT |
| M | 100 | 0 | 100 | 0 | NT | NT | |
| P | 100 | 0 | 100 | 0 | NT | NT | |
| Piperacillin/tazobactam | T | 100 | 83 | 100 | 50 | 100 | 33 |
| M | 100 | 33 | 100 | 0 | 100 | 0 | |
| P | 100 | 0 | 100 | 0 | 100 | 0 | |
T, trough level; M, mid level; P, peak level.
NT, not tested.
TABLE 3.
Percent recovery of various gram-positive pathogens for each system at each antimicrobial level
| Drug | Concna | % Recovery of organism with indicated systemb
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
S. aureus ATCC 25923
|
S. pneumoniae ATCC 49619
|
S. aureus ATCC 43300
|
Viridans streptococcus ATCC 10557
|
E. faecalis ATCC49533
|
E. faecium ATCC 35667
|
S. agalactiae ATCC 12385
|
|||||||||
| PLUS | FA | PLUS | FA | PLUS | FA | PLUS | FA | PLUS | FA | PLUS | FA | PLUS | FA | ||
| Vancomycin | P | 100 | 0 | 33 | 0 | 100 | 0 | 100 | 0 | 100 | 0 | 100 | 0 | NT | NT |
| M | 100 | 0 | 66 | 0 | 100 | 0 | 100 | 0 | 100 | 83 | 100 | 0 | NT | NT | |
| T | 100 | 83 | 100 | 0 | 100 | 83 | 100 | 17 | 100 | 100 | 100 | 33 | NT | NT | |
| Ampicillin | P | NT | NT | 100 | 0 | NT | NT | NT | NT | 100 | 0 | NT | NT | 100 | 0 |
| M | NT | NT | 100 | 0 | NT | NT | NT | NT | 100 | 0 | NT | NT | 100 | 0 | |
| T | NT | NT | 100 | 0 | NT | NT | NT | NT | 100 | 0 | NT | NT | 100 | 0 | |
| Oxacillin | P | 100 | 0 | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT |
| M | 100 | 0 | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | |
| T | 100 | 0 | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | |
| Piperacillin/tazobactam | P | 100 | 0 | 100 | 0 | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT |
| M | 100 | 0 | 100 | 0 | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | |
| T | 100 | 0 | 100 | 0 | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | |
| Cefoxitin | P | 100 | 0 | 100 | 0 | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT |
| M | 100 | 0 | 100 | 0 | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | |
| T | 100 | 0 | 100 | 100 | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | |
| Ceftriaxone | P | NT | NT | 0 | 0 | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT |
| M | NT | NT | 0 | 0 | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | |
| T | NT | NT | 0 | 0 | NT | NT | NT | NT | NT | NT | NT | NT | NT | NT | |
| Gentamicin/penicillin | P | NT | NT | NT | NT | NT | NT | NT | NT | 100 | 0 | NT | NT | NT | NT |
| M | NT | NT | NT | NT | NT | NT | NT | NT | 100 | 0 | NT | NT | NT | NT | |
| T | NT | NT | NT | NT | NT | NT | NT | NT | 100 | 100 | NT | NT | NT | NT | |
T, trough level; M, mid level; P, peak level.
NT, not tested; PLUS, BACTEC PLUS bottles; FA, BacT/Alert bottles.
For cultures with cefoxitin, overall recovery for the BACTEC PLUS system and the BacT/Alert system was 100% (96/96) and 38.5% (37/96) of strains, respectively. BACTEC PLUS recovered 100% of strains from control and test bottles at all levels of cefoxitin. With the BacT/Alert medium, 100% of strains were recovered from the control bottles, and 54.2%, 0%, and 0% of strains were recovered from test bottles at trough, mid, and peak levels of cefoxitin, respectively. The average times to detection were 10.2 h and 15.4 h with BACTEC and BacT/Alert media, respectively.
For cultures with vancomycin, overall recovery for the BACTEC PLUS system and the BacT/Alert system was 95.8% (138/144) and 37.5% (54/144) of strains, respectively. BACTEC PLUS recovered 100% of strains from control bottles and 94.4% of strains from test bottles, corresponding to 100% (36/36), 94.4% (34/36), and 88.9% (32/36) at trough, mid, and peak levels, respectively. For the BacT/Alert media, 100% of strains from the control bottles were recovered; 54.2%, 0%, and 0% of strains from the test bottles were recovered at trough, mid, and peak levels, respectively.
For cultures with cefepime, overall recovery for the BACTEC PLUS system and the BacT/Alert system was 88.9% (64/72) and 44.5% (32/72), respectively. BACTEC PLUS recovered 100% of strains from control bottles and 85.2% of strains from test bottles, with 100%, 88.9%, and 66.7% of challenge strains being recovered at trough, mid, and peak levels of cefepime, respectively. One hundred percent of the strains were recovered from the BacT/Alert control bottles; 66.7%, 11.1%, and 0% of strains were recovered from test bottles at trough, mid, and peak levels, respectively.
Neutralization of ampicillin and oxacillin was similar as indicated by the recovery of organisms. With ampicillin, the BACTEC PLUS system recovered 100% (72/72) of strains in control and test bottles. The BacT/Alert system recovered 25% (18/72) of all strains, including 100% (18/18) of strains from control bottles and 0% (0/54) of strains from test bottles. With oxacillin, the BACTEC PLUS system recovered 100% (24/24) of strains from control and test bottles. The BacT/Alert system recovered 25% (6/24) of all strains, including 100% (6/6) of strains from control bottles and 0% (0/24) of strains from test bottles.
A gentamicin/penicillin combination was also tested. The BACTEC PLUS system recovered 100% (24/24) of control and challenge strains. The BacT/Alert system recovered 50% of all strains, including 100% (6/6) of control strains and 33.3% (6/18) of challenge strains. Challenge strains were recovered at trough levels only.
Antibiotic levels at zero time and 1 h postinoculation were measured for vancomycin and cefoxitin (Table 4). After 1 h of incubation, the remaining amount of antibiotic was calculated. For vancomycin, with no vancomycin the remaining antibiotic was 0% for both systems, at trough levels of vancomycin the remaining antibiotic was 0% for BACTEC PLUS and 88% for BacT/Alert system, at mid levels the remaining antibiotic was 0% for BACTEC PLUS and 90% for the BacT/Alert system, and at peak levels the remaining antibiotic was 30% for BACTEC PLUS and 72% for the BacT/Alert system. For cefoxitin, with no cefoxitin the remaining antibiotic was 0% for both systems, at mid levels of cefoxitin the remaining antibiotic was 0% for BACTEC PLUS and 71% for the BacT/Alert system, and at peak levels the remaining antibiotic was 0% for BACTEC PLUS and 70% for the BacT/Alert system (Table 4).
TABLE 4.
Mean concentrations and percentages of remaining vancomycin and cefoxitin after 1 h of incubation in BACTEC PLUS and BacT/Alert FA bottles
| Amt and type of antibiotic at time zero | BACTEC PLUS concn [μg/ml (%)] | BacT/Alert FA concn [μg/ml (%)] |
|---|---|---|
| No vancomycin | 0 (0) | 0 (0) |
| Trough (10 μg/ml) vancomycin | 0 (0) | 8.8 (88) |
| Mid (25 μg/ml) vancomycin | 0 (0) | 22.5 (90) |
| Peak (50 μg/ml) vancomycin | 15 (30) | 36 (72) |
| No cefoxitin | <10 (0) | <10 (0) |
| Mid (60 μg/ml) cefoxitin | <10 (0) | 43 (71) |
| Peak (110 μg/ml) cefoxitin | <10 (0) | 77 (70) |
DISCUSSION
Numerous clinical studies comparing both the BACTEC PLUS and BacT/Alert media to standard blood culture bottle formulations for recovery of significant pathogens have been performed (3, 9, 10, 12, 13, 15, 18, 22, 23, 24). In most cases, the superiority of resin or charcoal-containing media for recovering clinically significant pathogens from adults and children with sepsis has been demonstrated (3, 9, 10, 12, 13, 15, 18, 22, 23, 24). The predecessors to the FA bottles, FAN bottles, contain brain heart infusion broth supplemented with Ecosorb, a proprietary substance that consists of Fuller's earth and activated carbon particles (24). In an initial study of the anaerobic FAN bottles versus standard anaerobic bottles, Wilson et al. (24) determined statistically significantly increased rates of recovery of S. aureus, coagulase-negative staphylococci, and E. coli, and higher overall rates of pathogen recovery in the FAN bottles, whereas the standard bottles recovered significantly more nonfermenters, Candida glabrata, and other yeasts (24). In terms of the speed of detection, the standard bottles were faster than FAN bottles in overall detection (P < 0.001). Similar results have been reported by investigators who evaluated the BacT/Alert standard aerobic and FAN aerobic blood culture bottles (10, 22), including the pediatric formulations. Enhanced recovery of coagulase-negative staphylococcal contaminants was reported in the study by Weinstein et al. (22). That study (22) did not stratify the data by antibiotic therapy at the time of blood draw. In the pediatric study, when the analysis was performed on cultures obtained from patients on antibiotics, the BacT/Alert PF bottle recovered more organisms than the Pedi-BacT system (P = 0.0180) (10).
In 1992, Marcelis et al. (12) reported that the BACTEC PLUS high-blood-volume resin medium grew significantly more S. aureus, coagulase-negative staphylococci, Candida albicans, E. faecalis, and P. aeruginosa than the standard bottles. After controlling for blood volume, enhanced recovery of S. aureus and C. albicans compared to that of standard BACTEC media was noted (12).
Few clinical studies have directly compared the BACTEC PLUS medium to the BacT/Alert FAN or FA medium. In a four-center study of adult patients with suspected sepsis, the BACTEC PLUS/F bottles were superior to the FAN bottles only in the recovery of Histoplasma capsulatum (P < 0.005) (9). The BACTEC system was faster (mean TTD, 16.9 h) than the BacT/Alert system (mean TTD, 18.7 h) in detecting positive blood cultures (9).
About 7 years ago, Organon Teknika introduced a new aerobic FAN blood culture medium designated BacT/ALERT FA. Substantial changes to this medium are described elsewhere (15), but they include changes in the medium formulations, greater headspace, total medium volume (30 ml instead of 40 ml), and a decrease in the concentration of activated charcoal from 8.5% (wt/vol) to 6.5% (wt/vol) (15). Mirrett et al. (15) compared both formulations in a study of adult patients with suspected sepsis. The FA bottle detected more Burkholderia cepacia, C. albicans, and Cryptococcus neoformans isolated and all microorganisms combined than the FAN bottles. In the subset of patients on antimicrobial therapy, FAN bottles were superior to FA bottles in detecting S. aureus (P < 0.005), whereas FA bottles were superior in recovering yeasts (P < 0.005). The times to detection of bottles giving positive results were the same overall between the two systems (15).
Finally, at least one study has demonstrated the superiority of FAN bottles over standard media for detecting clinically important episodes of bacteremia from all patients. Among patients receiving antimicrobial agents there was a twofold increase in recovery of clinically significant bacteremia using FAN compared to standard bottles (13). All of the above studies suggest that the superiority of media containing antibiotic removal devices compared to standard media is probably related to factors other than antibiotic neutralization.
Few studies have evaluated the effectiveness of the resin or charcoal-containing blood culture media in neutralizing the effects of antimicrobials. Nzeako et al. (16) completed a study analyzing the ability of BACTEC PLUS media to remove vancomycin, amoxicillin, chloramphenicol, penicillin, amphotericin B, gentamicin, and amikacin. The investigators tested antibiotic concentrations based on serial dilutions from 0.8 μg/ml to 200 μg/ml. They also challenged the system with an antifungal agent and the ability to grow C. albicans. They concluded that the BACTEC PLUS medium is capable of neutralizing the effect of antibiotics up to a concentration of 100 μg/ml (a concentration higher than any blood level found clinically).
A study completed by Vigano et al. (21) analyzed the ability of both the BACTEC PLUS and BacT/Alert FAN media to remove ampicillin, cefotaxime, gentamicin, vancomycin, ciprofloxacin, and trimethoprim-sulfamethoxazole. The time to detection of frequently encountered bacteria was evaluated with antibiotics at trough and higher concentrations. In that study, the BACTEC PLUS system recovered more pathogens with shorter times to detection than the BacT/Alert FAN system when beta-lactams were present at concentrations corresponding to trough levels during parenteral therapy. The two systems seemed to be equally efficient when gentamicin, ciprofloxacin, and trimethoprim-sulfamethoxazole were tested. With vancomycin, the BACTEC PLUS system seemed more efficient than the BacT/Alert FAN system. This study was similar to ours as it simulated clinical practice conditions and variables such as blood volume, bacterial load, antibiotic concentration, and timing were controlled. It differed from our evaluation as the authors also determined the MICs of the antibiotics against the tested organisms. They determined that organisms could be inhibited by antibiotic levels lower than trough levels and concluded that the use of an antibiotic removal device was of paramount importance.
Spaargaren et al. (19) analyzed the effectiveness of resins in neutralizing antibiotics by determining antibiotic concentrations over time. Utilizing high-performance liquid chromatography and competitive immunological methods (TDx; Abbott Diagnostics, Abbott Park, IL), levels of flucloxacillin, cefamandole, sulfamethoxazole, trimethoprim, gentamicin, and teicoplanin were tested at 0, 0.5, 1, 2, 4, and 6 h. After 1 h of incubation in the resin medium, the levels of antibiotics were reduced to 17%, 38%, 5%, 0%, 20%, and 59%, respectively. These findings are similar to our results for vancomycin and cefoxitin, with levels reduced to 30% and 0%, respectively, at peak levels for BACTEC versus BacT/Alert after 1 h of incubation.
Our study utilized antibiotic concentrations that correlated with therapeutic serum levels. We chose organisms that are typically associated with bacteremia and that are probably being treated with the antibiotics administered in our hospital. A positive blood culture allows for the rapid recovery and identification of the causative agent of bacteremia, increasing the likelihood of appropriate therapy and improved outcomes. Both systems performed well in the presence of gentamicin. Neither system was able to neutralize the effects of ceftriaxone when the fastidious S. pneumoniae was the test organism. However, there were marked differences between the two systems with respect to the rates of bacterial detection for the remaining organism-drug combinations. Overall, the BACTEC PLUS system recovered 95.1% of the bacterial isolates, while the BacT/Alert system recovered 43.1% of the bacterial isolates. This study demonstrates the superiority of the BACTEC PLUS medium compared to the BacT/Alert FAN system in recovering gram-positive and gram-negative pathogens in the presence of frequently used antibiotics.
This study has implications for clinical laboratories and institutions where a significant proportion of blood cultures from patients are carried out while the patient is receiving therapy.
As antimicrobial agents change, it is important to reevaluate the efficacy of these systems. The present study is the first in vitro study completed in the United States to compare the ability of the two systems to neutralize the effects of antimicrobial agents. Our study supports those previously performed in Europe (19, 21) and indicates that there are differences in recovery and time to detection of significant pathogens depending upon the antibiotic removal agents present in the blood culture media. In our study, the BACTEC PLUS medium was superior to the BacT/Alert FA medium in its ability to neutralize antimicrobial agents and allow bacterial recovery. Assuming that the binding of antimicrobial agents contributes in part to enhanced performance of these bottles and superiority over standard media for establishing a diagnosis of significant bacteremia in patients receiving antimicrobial therapy (13), it is important that hospitals using the BacT/Alert system emphasize to clinicians that the optimum time to obtain blood cultures from these patients is at the trough level (that is, just prior to the next dose of antimicrobial agent). For hospitals using the BACTEC PLUS system, the timing for collection of blood cultures is not as critical for optimal recovery of pathogens due to the efficient binding of antibiotics by the resins in the medium.
Acknowledgments
We acknowledge the following staff and microbiologists for their contribution to the study and manuscript preparation: all members of The Johns Hopkins Microbiology Laboratory, the Special Chemistry Laboratory at The Johns Hopkins Hospital, and Paula Johnson and Mike Borlet from BD Diagnostics, Inc. We also thank Veronica Kemp for completing the vancomycin level testing.
This study was supported in part by BD Diagnostics, Sparks, MD.
Footnotes
Published ahead of print on 13 December 2006.
REFERENCES
- 1.Amsden, G. W. 2000. Tables of antimicrobial pharmacology, p. 551-601. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 5th ed. Churchill Livingstone, Philadelphia, PA.
- 2.Anhalt, J. P. 1981. Antimicrobial assays, p. 684-701. In J. A. Washington II (ed.), Laboratory procedures in clinical microbiology. Springer-Verlag, New York, NY.
- 3.Appelbaum, P. C., D. G. Beckwith, J. R. Dipersio, J. W. Dyke, J. F. Salventi, and L. L. Stone. 1983. Enhanced detection of bacteremia with a new BACTEC resin blood culture medium. J. Clin. Microbiol. 17:48-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appleman, M. D., R. S. Swinney, and P. N. Heseltine. 1982. Evaluation of the Antibiotic Removal Device. J. Clin. Microbiol. 15:278-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baddour, L. M., W. R. Wilson, A. S. Bayer, V. G. Fowler, Jr., A. F. Bolger, M. E. Levison, P. Ferrieri, M. A. Gerber, L. Y. Tani, M. H. Gewitz, D. C. Tong, J. M. Steckelberg, R. S. Baltimore, S. T. Shulman, J. C. Burns, D. A. Falace, J. W. Newberger, T. J. Pallasch, M. Takahashi, K. A. Taubert; Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease; Council on Cardiovascular Disease in the Young; Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia; American Heart Association; Infectious Diseases Society of America. 2005. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 111:e394-434. [DOI] [PubMed] [Google Scholar]
- 6.Carleton, J., and M. Hamburger. 1963. Unmasking of false-negative blood cultures in patients receiving new penicillins. JAMA 186:157-159. [DOI] [PubMed] [Google Scholar]
- 7.Crepin, O., M. Roussel-Delvallez, G. R. Martin, and R. J. Courcol. 1993. Effectiveness of resins in removing antibiotics from blood cultures. J. Clin. Microbiol. 31:734-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dowling, H. F., and H. L. Hirsh. 1945. The use of penicillinase in cultures of body fluids obtained from patients under treatment with penicillin. Am. J. Med. Sci. 210:756-762. [DOI] [PubMed] [Google Scholar]
- 9.Jorgensen, J. H., S. Mirrett, L. C. McDonald, P. R. Murray, M. P. Weinstein, J. Fune, C. W. Trippy, M. Masterson, and L. B. Reller. 1997. Controlled clinical laboratory comparison of BACTEC Plus aerobic/F resin medium with BacT/Alert aerobic FAN medium for detection of bacteremia and fungemia. J. Clin. Microbiol. 35:53-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krisher, K. K., P. Gibb, S. Corbett, and D. Church. 2001. Comparison of the BacT/Alert PF pediatric FAN blood culture bottle with the standard pediatric blood culture bottle, the Pedi-BacT. J. Clin. Microbiol. 39:2880-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magadia, R. R., and M. P. Weinstein. 2001. Laboratory diagnosis of bacteremia and fungemia. Infect. Dis. Clin. N. Am. 15:1009-1024. [DOI] [PubMed] [Google Scholar]
- 12.Marcelis, L., J. Verhaegen, J. Vandeven, A. Bosmans, and L. Verbist. 1992. Evaluation of Bactec high blood volume resin media. Diagn. Microbiol. Infect. Dis. 15:385-391. [DOI] [PubMed] [Google Scholar]
- 13.McDonald, L. C., J. Fune, L. B. Gaido, M. P. Weinstein, L. G. Reimer, T. M. Flynn, M. L. Wilson, S. Mirrett, and L. B. Reller. 1996. Clinical importance of increased sensitivity of BacT/Alert FAN aerobic and anaerobic blood culture bottles. J. Clin. Microbiol. 34:2180-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGuire, N. M., C. A. Kauffman, C. S. Hertz, and J. M. Kovach. 1983. Evaluation of the BACTEC antimicrobial removal system for detection of bacteremia. J. Clin. Microbiol. 18:449-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mirrett, S., R. J. Everts, and L. B. Reller. 2001. Controlled comparison of original vented aerobic FAN medium with new nonvented BacT/ALERT FA medium for culturing blood. J. Clin. Microbiol. 39:2098-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nzeako, B. C., and S. S. H. Al-Qasabi. 2004. Evaluation of the neutralising capacity of Bactec medium for some antibiotics. Br. J. Biomed. Sci. 61:171-174. [DOI] [PubMed] [Google Scholar]
- 17.Pittet, D. 1997. Nosocomial bloodstream infections, p. 711-769. In R. P. Wenzel (ed.), Prevention and control of nosocomial infections, 3rd ed. Williams and Wilkins, Baltimore, MD.
- 18.Pohlman, J. K., B. A. Kirkley, K. A. Easley, B. A. Basille, and J. A. Washington. 1995. Controlled clinical evaluation of BACTEC Plus Aerobic/F and BacT/Alert Aerobic FAN bottles for detection of bloodstream infections. J. Clin. Microbiol. 33:2856-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spaargaren, J., C. P. A. van Boren, and G. P. Voorn. 1998. Effectiveness of resins in neutralizing antibiotic activities in Bactec Plus Aerobic/F culture medium. J. Clin. Microbiol. 36:3731-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomson Healthcare. 2006. 2006 Physician's Desk Reference, 60th ed. Piperacillin/tazobactam, p. 3492-3493. Thomson Healthcare, Inc., Montvale, NJ.
- 21.Vigano, E. F., E. Vasconi, C. Agrappi, P. Clerici, and P. Melloni. 2004. Use of simulated blood cultures for antibiotic effect on time to detection of the two blood culture systems BacT/Alert and Bactec 9240. New Microbiol. 27:235-248. [PubMed] [Google Scholar]
- 22.Weinstein, M. P., S. Mirrett, L. G. Reimer, M. L. Wilson, S. Smith-Elekes, C. R. Chuard, K. L. Joho, and L. B. Reller. 1995. Controlled evaluation of BacT/Alert standard aerobic and FAN aerobic blood culture bottles for detection of bacteremia and fungemia. J. Clin. Microbiol. 33:978-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinstein, M. P., M. L. Towns, S. M. Quartey, S. Mirrett, L. G. Reimer, G. Parmigiani, and L. B. Reller. 1997. The clinical significance of positive blood cultures in the 1990s: a prospective, comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin. Infect. Dis. 24:584-602. [DOI] [PubMed] [Google Scholar]
- 24.Wilson, M. L., M. P. Weinstein, S. Mirrett, L. G. Reimer, R. J. Feldman, C. R. Chuard, and L. B. Reller. 1995. Controlled evaluation of BacT/Alert standard anaerobic and FAN anaerobic blood culture bottles for the detection of bacteremia and fungemia. J. Clin. Microbiol. 33:2265-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright, A. J., R. L. Thompson, C. A. McLimans, W. R. Wilson, and J. A. Washington II. 1982. The antimicrobial removal device. A microbiological and clinical evaluation. Am. J. Clin. Pathol. 78:173-177. [DOI] [PubMed] [Google Scholar]