Abstract
In the present study, novel real-time PCR assays targeting the fungal ITS2 region were developed for the detection and differentiation of medically important Aspergillus species (Aspergillus fumigatus, Aspergillus flavus, Aspergillus nidulans, Aspergillus niger, and Aspergillus terreus) and Candida species (Candida albicans, Candida dubliniensis, Candida glabrata, Candida krusei, Candida parapsilosis, and Candida tropicalis) using a LightCycler instrument. The combination of a group-specific and a universal primer with five Aspergillus or six Candida species-specific biprobes in one reaction mixture facilitated rapid screening and species differentiation by the characteristic peak melting temperatures of the biprobes. Both assays can be performed either as single assays or simultaneously in the same LightCycler run. The analytical sensitivity using pure cultures and EDTA-anticoagulated blood, cerebrospinal fluid (CSF), and tissue samples spiked with A. fumigatus and C. albicans cell suspensions was shown to be at least 1 CFU per PCR, corresponding to 5 to 10 CFU/ml blood and 10 CFU/200 μl CSF or 0.02 g tissue. To assess the clinical applicability, 26 respiratory samples, 4 tissue samples from the maxillary sinus, and 1 blood sample were retrospectively tested and real-time PCR results were compared with results from culture, histology, or a galactomannan enzyme-linked immunosorbent assay (ELISA). Twenty samples (64.5%) were both culture positive and positive by real-time PCR. Six samples (19.4%) showed no growth of fungi but were positive by real-time PCR. However, all of the tissue samples were positive by both PCR and histology. The blood sample showed no growth of Aspergillus, but aspergillosis was confirmed by positive galactomannan ELISA, histology, and PCR results. The remaining samples (16.1%) were culture and PCR negative; also, no other signs indicating fungal infection were observed. Our data suggest that the Aspergillus and Candida assays may be appropriate for use in clinical laboratories as simple and rapid screening tests for the most frequently encountered Aspergillus and Candida species and might become an important tool in the early diagnosis of fungal infections in the future.
Invasive fungal infections are increasingly recognized as a primary cause of morbidity and mortality, especially in immunocompromised patients. The frequency of nosocomial candidemia has increased 10-fold during the past two decades (3). Candida albicans, formerly the most important species, is still the one which most often causes disease. However, other species than C. albicans, in particular, Candida glabrata, but also Candida tropicalis, Candida krusei, and Candida parapsilosis, have gained greater significance and must not be overlooked (15, 31). The prevalence of candidiasis and the increase in Candida being resistant to polyene and azole drugs have made rapid species differentiation mandatory (29, 31). There has been a less striking, though also substantial, increase in the incidence of invasive aspergillosis (IA) (27). Invasive aspergillosis is mainly caused by Aspergillus fumigatus, followed by Aspergillus flavus and Aspergillus terreus. Other species, such as Aspergillus nidulans, Aspergillus niger, and Aspergillus ustus, are rarely found in diagnosing IA.
The definite and rapid diagnosis of invasive fungal infections is difficult due to the lack of sensitive test methods. Therefore, efforts to improve diagnosis are ongoing and need to be further intensified. Although proving the presence of infection by histology and culture remains the cornerstone of diagnosis, non-culture-based methods are being developed to allow early detection. Among the most promising approaches are the detection of fungal antigens and PCR (2, 4, 42). The results of some studies suggest that a combination of the Aspergillus galactomannan enzyme-linked immunosorbent assay (GM-ELISA) and real-time PCR may provide improved diagnosis of invasive aspergillosis (5, 7, 20, 35).
Nevertheless, not only the detection of fungi, but also their identification, which can be obtained only by PCR or culture, is important for the optimal choice of antifungals and duration of therapy. A variety of PCR assays based on the detection of fungal DNA in sterile human body fluids or tissue samples to allow early diagnosis of fungal infections and to improve the survival rate of patients suffering from invasive infections has been described. However, in contrast to the GM-ELISA, none of the developed PCR assays have been standardized, resulting in diverging results. In general, the introduction of real-time PCR technology in the detection of fungal infections has increased the reliability of PCR results compared to results obtained by conventional PCR methods. Real-time PCR sharply decreases the risk of false-positive results due to PCR product carryover during gel electrophoresis with subsequent Southern blot hybridization or enzyme-linked immunoassays to check the specificity of the PCR product. The identification of species via melting curve analysis with species-specific hybridization probes further increases specificity, as one mismatch in the probe binding site would lead to an altered melting temperature (Tm). The fast turnaround time of less than 2 h is another advantage of the real-time PCR technology (4, 13, 14).
Although infections with Candida and Aspergillus species are the ones most encountered by high-risk patients, there are only a few reports of PCR assays specifically targeting Candida spp. and Aspergillus spp. at the same time (21, 32). Furthermore, the majority of Aspergillus-specific real-time PCR assays are restricted to the detection of A. fumigatus alone. Therefore, we have decided to design a real-time PCR approach which enables culture-independent screening for Candida and Aspergillus infections within only a few hours. The aim of this study was to develop assays specific for the fungal ITS2 region, allowing the simultaneous detection and differentiation of 11 medically important species of Aspergillus (Aspergillus fumigatus, Aspergillus flavus, Aspergillus nidulans, Aspergillus niger, and Aspergillus terreus) and Candida (Candida albicans, Candida dubliniensis, Candida glabrata, Candida krusei, Candida parapsilosis, and Candida tropicalis) with species-specific biprobes on the LightCycler instrument (Roche Diagnostics GmbH, Mannheim, Germany). Biprobes were chosen as they guarantee high specificity and have not yet been applied to the species differentiation of fungi.
MATERIALS AND METHODS
Fungal strains and DNA extraction.
Fungal strains were obtained from the American Type Culture Collection (ATCC), Manassas, VA; the Centraalbureau voor Schimmelcultures (CBS), Utrecht, The Netherlands; or the Institute of Hygiene and Medical Microbiology, Medical University of Vienna. The identification of all clinical isolates was confirmed by conventional morphological and physiological methods (1, 40). DNA was extracted from the following isolates: C. glabrata (ATCC 90030), C. tropicalis (ATCC 750), C. albicans (ATCC 44374 and 10 clinical isolates), C. parapsilosis (ATCC 22019), C. krusei (ATCC 6258 and 2 clinical isolates), C. dubliniensis (4 clinical isolates), Candida kefyr (clinical isolate), Cryptococcus neoformans (ATCC 62006), Malassezia furfur (clinical isolate), Malassezia pachydermatis (clinical isolate), Trichosporon sp. (clinical isolate), Saccharomyces cerevisiae (ATCC 9763), A. flavus (ATCC 64025), A. niger (ATCC 10578 and 9 clinical isolates), A. fumigatus (ATCC 14110 and 3 clinical isolates), A. terreus (CBS 116.46 and 5 clinical isolates), A. nidulans (3 isolates obtained from an external quality control program; UK NEQAS, Central Public Health Laboratory, London, United Kingdom), and clinical isolates of Aspergillus sydowii, Aspergillus versicolor, Aspergillus ustus, Penicillium spp., Rhizopus oryzae, Mucor sp., Rhizomucor pusillus, Absidia corymbifera, Cunninghamella sp., Syncephalastrum sp., Scedosporium apiospermum, Fusarium sp., Verticillium sp., and Phialophora sp.
Fungal isolates were cultured by standard cultivation methods. Cell suspensions were prepared with 0.9% saline and adjusted to a 3 McFarland standard. For quantification of the Candida and Aspergillus suspensions, 10-fold serial dilutions were plated and CFU were counted. The fungal suspensions were centrifuged, resuspended in 200 μl 0.9% NaCl, and incubated with 20 U recombinant Lyticase (Sigma-Aldrich, Austria) at 37°C for 30 min. DNA was extracted with a High Pure PCR template preparation kit (Roche Molecular Biochemicals, Mannheim, Germany) by following the instructions of the manufacturer. DNA was eluted with 100 μl elution buffer provided with the kit.
Clinical samples.
Samples obtained for routine microbiology diagnostic procedures from patients suspected and not suspected of having fungal infections were retained for evaluation of the PCR assays. Bronchoalveolar lavage (BAL) (n = 19), bronchial secretion (n = 5), and tracheal secretion (n = 2) samples and an EDTA-anticoagulated blood sample (n = 1) from lung transplant recipients and from patients in the intensive care unit were used for further investigations. Tissue samples from the maxillary sinus (n = 4) were obtained from patients with histologically proven fungus balls. Part of the material was cultured using standard cultivation methods. The remaining material (1 to 2 ml) was stored at −20°C until it was used for DNA extraction.
DNA extraction from 3 ml EDTA-blood sample.
A modification of a protocol described by Loeffler et al. (25) was used. For red cell lysis, 3 ml EDTA-blood was mixed with 15 ml lysis buffer (LB; 10 mM Tris [pH 7.6], 5 mM MgCl2, 10 mM NaCl), incubated for 15 min on ice, and then centrifuged for 10 min at 3,000 rpm. The pellet was resuspended in 15 ml LB, incubated again for 15 min on ice, and then centrifuged for 10 min at 3,000 rpm. For white cell lysis, the pellet was then resuspended in 1 ml LB containing 200 μg/ml protease (QIAGEN, Hilden, Germany), incubated at 65°C for 45 min, and then centrifuged at 13,000 rpm for 10 min. In order to obtain spheroplasts, the pellet was resuspended in 500 μl Lyticase solution (50 mM Tris [pH 7.6], 1 mM EDTA [pH 8.0], 0.2% 2-mercaptoethanol) containing 20 U recombinant Lyticase, incubated at 37°C for 30 min, and then centrifuged at 13,000 rpm for 10 min. Finally, DNA was extracted with a High Pure PCR template preparation kit by following the instructions of the manufacturer. DNA was eluted with 100 μl elution buffer.
DNA extraction from 200 μl cerebrospinal fluid (CSF), BAL, bronchial, or tracheal secretion samples.
For concentration of the fungi, 1 to 2 ml of the specimen was centrifuged for 5 min at 13,000 rpm. In order to obtain spheroplasts, the pellet plus 200 μl supernatant was incubated with 20 U recombinant Lyticase at 37°C for 30 min. Finally, DNA was extracted with a High Pure PCR template preparation kit by following the instructions of the manufacturer. DNA was eluted with 100 μl elution buffer.
DNA extraction from tissue samples.
Tissue (0.02 g) was incubated in 200 μl elution buffer and 200 μl binding buffer from the High Pure PCR template preparation kit with 900 μg protease at 55°C until the tissue was completely digested. After inactivation of the protease (95°C for 5 min), the sample was treated with 20 U recombinant Lyticase at 37°C for 30 min to obtain spheroplasts. Finally, DNA was extracted with a High Pure PCR template preparation kit by following the instructions of the manufacturer. DNA was eluted with 100 μl elution buffer.
In order to avoid contamination, all steps were performed with aerosol-resistant tips. DNA extraction, preparation of the master mix, and addition of the template were carried out in two separate rooms. For each extraction, a reagent blank was carried out to exclude false-positive PCR results due to contamination.
Aspergillus- and Candida-specific biprobe assays.
GenBank was searched for sequences of the ITS2 regions of Candida and Aspergillus species and phylogenetically related fungi. The published sequences were aligned using ClustalW (http://www.ebi.ac.uk/clustalw/), and primers and probes were designed. A BLAST search (http://www.ncbi.nlm.nih.gov/BLAST/) was performed to check the specificity of the DNA sequences of the primers and probes. The sequences of the primers and probes are shown in Table 1. Primer Asp-F is mainly specific for members of the Hyphomycetes, whereas primer Cand-F is mainly specific for members of the yeasts. Both primers anneal within the ITS2 region. Primer ITS-R anneals to a highly conserved region of the 28S rRNA gene. For the detection and differentiation of the five Aspergillus species and the six Candida species, 11 different species-specific biprobes were designed, allowing species identification by specific melting peaks. Sequence alignments of the corresponding Aspergillus and Candida species are shown in Fig. 1 and 2, respectively.
TABLE 1.
Sequences and concentrations of primers and probes and corresponding probe-specific Tm in real-time PCR assays
| Primer or probe | Sequence (concn) | Probe-specific Tm |
|---|---|---|
| Candida-specific primers | ||
| Cand-F | CCTGTTTGAGCGTCRTTT (0.15 μM) | |
| ITS-R | TCCTCCGCTTATTGATAT (0.5 μM) | |
| Candida-specific probes | ||
| C.alb-S | Cy5-CATTGCTTGCGGCGGTA-biotin (0.2 μM) | 66°C or 55°C |
| C.glab-S | Cy5-GTTTTACCAACTCGGTGTTGAT-biotin (0.2 μM) | 65°C |
| C.trop-S | Cy5-GGCCACCACAATTTATTTCA-biotin (0.2 μM) | 63°C |
| C.krus-S | Cy5-CGAGCGAACTAGACTTT-biotin (0.2 μM) | 60°C |
| C.para-S | Cy5-GAAAGGCGGAGTATAAAC-biotin (0.2 μM) | 58°C |
| C.dubl-S | Cy5-CATTGCTAAGGCGGTCT-biotin (0.2 μM) | 62°C |
| Aspergillus-specific primers | ||
| Asp-F | CTGTCCGAGCGTCATTG (0.15 μM) | |
| ITS-R | TCCTCCGCTTATTGATAT (0.5 μM) | |
| Aspergillus-specific probes | ||
| A.fum-S | Cy5-AGCCGACACCCAACTTTATTT-biotin (0.2 μM) | 63°C |
| A.terr-S | Cy5-ACGCATTTATTTGCAACTTGTTTT-biotin (0.2 μM) | 57°C or 66°C |
| A.nidu-S | Cy5-GTCACCCGCTCGATTAGG-biotin (0.2 μM) | 66°C |
| A.nig-S | Cy5-TCACATGCTCTGTAGGATTGGCC-biotin (0.2 μM) | 68°C |
| A.flav-S | Cy5-TGCCGAACGCAAATCAAT-biotin (0.2 μM) | 59°C |
FIG. 1.
ClustalW multiple-sequence alignment of the ITS2 region amplified with primers Asp-F and ITS-R from A. fumigatus (AY373851), A. flavus (AJ876522), A. nidulans (AY373888), A. niger (AY373852), and A. terreus (A. terreus 1, AY373871; A. terreus 2, AJ001333; and A. terreus 3, AJ001368) (designations in parentheses are GenBank accession numbers). Incomplete sequences of the ITS2 regions of A. terreus 2 and A. terreus 3 were found in the database. Nucleotides corresponding to the species-specific biprobes are bold, in italics, and underlined.
FIG. 2.
ClustalW multiple-sequence alignment of the ITS2 region amplified with primers Cand-F and ITS-R from C. albicans (C. albicans 1, AY139782, and C. albicans 2, clinical isolate), C. dubliniensis (AY382338), C. glabrata (AY139784), C. krusei (AY235807), C. parapsilosis (AY217022), and C. tropicalis (AF321539) (designations in parentheses are GenBank accession numbers). Nucleotides corresponding to the species-specific biprobes are bold, in italics and underlined.
To facilitate rapid screening for the presence of Candida or Aspergillus in a sample, the six Candida-specific biprobes or the five Aspergillus-specific biprobes were used together in one reaction mixture. The 20-μl real-time PCR mixtures were prepared with 2 μl of LightCycler-FastStart DNA master SYBR green I (Roche Molecular Biochemicals, Mannheim, Germany), 4 mM MgCl2, primers and probes as shown in Table 1, and 3 μl of DNA extract made up to 20 μl with water. PCRs were performed in a LightCycler instrument with preliminary denaturation for 10 min at 95°C, followed by 60 amplification cycles (with a temperature transition rate of 20°C/s) of denaturation at 95°C for 8 s, annealing at 55°C for 10 s, and primer extension at 72°C for 10 s, with a single fluorescence acquisition step at the end of the extension. This was followed by a melting analysis of the probe-PCR product duplex consisting of 95°C for 30 s and then cooling to 35°C for 60 s before the temperature was raised to 98°C at a rate of 0.2°C/s with continuous fluorescence acquisition. A final cooling step was performed at 40°C for 10 s.
TABLE 3.
Culture and real-time PCR results for clinical specimens
| Patient | Specimen type | Culture result(s) | Real-time PCR
|
||
|---|---|---|---|---|---|
| Result or peak Tm(s) in:
|
Species confirmedc (peak Tm[s]) | ||||
| Aspergillus screeninga | Candida screeningb | ||||
| 1 | BAL | C. glabrata | Negative | 65°C | C. glabrata (65°C) |
| 2 | BAL | C. albicans | Negative | 56°C and 66°C | C. albicans (56°C and 66°C) |
| 3 | BAL | C. albicans | 52°C | 66°C | C. albicans (66°C) |
| 4 | BAL | C. albicans and C. tropicalis | Negative | 63°C | C. albicans (66°C) and C. tropicalis (63°C) |
| 5 | BAL | C. albicans and C. tropicalis | Negative | 64°C | C. albicans (66°C) and C. tropicalis (63°C) |
| 6 | BAL | C. dubliniensis and C. glabrata | Negative | 64°C | C. dubliniensis (63°C) and C. glabrata (65°C) |
| 7 | Bronchial secretion | A. fumigatus and C. albicans | 63°C | 66°C | A. fumigatus (63°C) and C. albicans (66°C) |
| 8 | Tracheal secretion | A. fumigatus and C. albicans | 63°C | 66°C | A. fumigatus (63°C) and C. albicans (66°C) |
| 9 | BAL | C. glabrata | Negative | 65°C | C. glabrata (65°C) and C. albicans (66°C) |
| 10 | BAL | C. glabrata | Negative | 64°C | C. glabrata (65°C) and C. albicans (66°C) |
| 11 | BAL | C. tropicalis | 52°C | 65°C | C. tropicalis (63°C) and C. albicans (66°C) |
| 12 | Bronchial secretion | A. fumigatus | 63°C | 66°C | A. fumigatus (63°C) and C. albicans (66°C) |
| 13 | Bronchial secretion | A. fumigatus | 63°C | 66°C | A. fumigatus (63°C) and C. albicans (66°C) |
| 14 | Bronchial secretion | A. fumigatus | 63°C | 66°C | A. fumigatus (63°C) and C. albicans (66°C) |
| 15 | BAL | A. fumigatus and A. flavus | 59°C | 66°C | A. fumigatus (63°C), A. flavus (59°C), and C. albicans (66°C) |
| 16 | Bronchial secretion | A. fumigatus | 63°C | 65°C | A. fumigatus (63°C), C. albicans (66°C), and C. glabrata (65°C) |
| 17 | BAL | C. lusitaniae | Negative | 65°C | C. glabrata (65°C) |
| 18 | BAL | A. niger and C. albicans | 68°C | Negative | A. niger (68°C) |
| 19 | BAL | C. dubliniensis, C. glabrata, C. albicans, and C. tropicalis | Negative | 63°C | C. dubliniensis (63°C) and C. glabrata (65°C) |
| 20 | BAL | A. flavus and A. fumigatus | 59°C | Negative | A. flavus (59°C) |
| 21 | Fungus balld | Negative | 63°C | Negative | A. fumigatus (63°C) |
| 22 | Fungus balld | Negative | 63°C | Negative | A. fumigatus (63°C) |
| 23 | Fungus balld | Negative | 63°C | Negative | A. terreus (63°C) |
| 24 | Fungus balld | Negative | 63°C | 63°C | A. fumigatus (63°C) and C. tropicalis (63°C) |
| 25 | Tracheal secretion | Negative | Negative | 55°C and 66°C | C. albicans (55°C and 66°C) |
| 26 | Blood | Negative | 59°C | Negative | A. flavus (59°C) |
| 27 | BAL | Negative | Negative | Negative | Negative |
| 28 | BAL | Negative | 52°C | Negative | Negative |
| 29 | BAL | Negative | Negative | Negative | Negative |
| 30 | BAL | Negative | Negative | Negative | Negative |
| 31 | BAL | Negative | Negative | Negative | Negative |
Screening was performed with five Aspergillus-specific biprobes in one PCR mixture.
Screening was performed with six Candida-specific biprobes in one PCR mixture.
Species confirmation was performed with only the appropriate species-specific biprobe in the PCR mixture.
The identification was proven by histology.
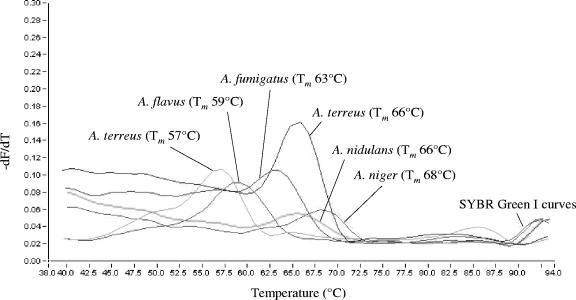
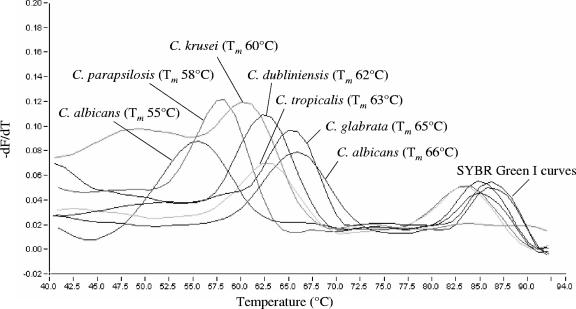
Light emission due to SYBR green was monitored in the F1 channel, whereas biprobe-specific melting peaks were analyzed in the F3 channel of the instrument. Samples were considered positive for an Aspergillus or Candida species upon the presence of a biprobe-specific melting peak. In this case, the species was confirmed by repeating the analysis with the appropriate specific biprobe in the reaction mixture. Specific Tm values of the biprobes are shown in Table 1. Melting peaks obtained with the Aspergillus and Candida species-specific biprobes are shown in Fig. 3 and 4, respectively.
FIG. 3.
Melting peaks obtained from A. fumigatus, A. flavus, A. nidulans, A. niger, and A. terreus with the corresponding species-specific biprobes. The values on the y axis are the first negative derivative of the change in fluorescence (dF) divided by the change in temperature (dT) (−dF/dT).
FIG. 4.
Melting peaks obtained from C. albicans, C. dubliniensis, C. glabrata, C. krusei, C. parapsilosis, and C. tropicalis with the corresponding species-specific biprobes. The values on the y axis are the first negative derivative of the change in fluorescence (dF) divided by the change in temperature (dT) (−dF/dT).
The detection of galactomannan in the blood sample (patient 26) was performed by means of the Platelia Aspergillus enzyme immunoassay (EIA) kit (Bio-Rad Laboratories Central Europe, Vienna, Austria), according to the manufacturer's instructions.
Evaluation of analytical sensitivity and specificity.
In order to evaluate the analytical sensitivity of the assays, dilutions of DNA of the 11 species in the range between 105 and 10−1 CFU per PCR were used as template DNA. For evaluation of the DNA extraction protocols, 3 ml EDTA-blood, 200 μl CSF, and 0.02 g tissue (aorta) of noninfected patients or healthy volunteers were spiked with dilutions of C. albicans and A. fumigatus in the range between 104 and 100 CFU/ml. DNA was extracted and analyzed. To confirm the absence of PCR inhibitors, one additional reaction mixture containing the DNA extract was spiked with 4 CFU of C. albicans or A. fumigatus to exclude inhibition. The analytical specificity of the assays was evaluated with DNA extracted from Aspergillus, Candida, and all the other fungal isolates described. Furthermore, BLAST analyses of primers and probes were performed.
RESULTS
Specificity of the Aspergillus-specific biprobes and melting temperature values.
A BLAST search was performed to check the specificity of the Aspergillus-specific biprobes. Biprobes specific for A. flavus, A. fumigatus, A. nidulans, and A. niger had 100% sequence similarity to each of the respective Aspergillus strains in the database. They further revealed 100% similarity to some other Aspergillus, Emericella, and Neosartorya species, which may lead to cross-reactions (Table 2). The biprobe specific for A. terreus showed mismatches with three A. terreus strains (Fig. 1). To further prove the specificity, DNA of all fungal isolates mentioned under “Fungal strains and DNA extraction” was tested with each of the Aspergillus species-specific biprobes. As expected, cross-reactivity of biprobe A.nid-S was observed with A. sydowii, A. ustus, and A. versicolor. No cross-reactivity was observed with the other isolates. The Tm values of the biprobe-specific melting peaks are shown in Table 1 and Fig. 3. A. terreus strain CBS 116.46 revealed a biprobe-specific melting peak with a Tm value of 57°C instead of 66°C. The PCR product of this strain was sequenced and revealed the same sequence variation as the two strains with accession numbers AJ001333 and AJ001331 found in the database. Occasionally, an indistinct and broad peak at the 52°C Tm was observed when the five Aspergillus species-specific biprobes were used together for screening (Table 3).
TABLE 2.
Results of BLAST analyses of the Aspergillus and Candida species-specific biprobes
| Biprobe | No. with 100% sequence similarity to biprobe/total no. in GenBank database | Other species with 100% sequence similarity |
|---|---|---|
| A.flav-S | 29/29 A. flavus strains | A. oryzae, A. parasiticus, and Aureobasidium mansoniia |
| A.fum-S | 25/25 A. fumigatus strains | Aspergillus fumisynnematus, Aspergillus lentulus, Aspergillus viridinutans, Neosartorya aureola, Neosartorya botucatensis, Neosartorya fischeri, Neosartorya glabra, Neosartorya spinosa, and Neosartorya udagawae |
| A.nid-S | 14/14 A. nidulans strains | Emericella astellata, Emericella indica, Emericella quadrilineata, Emericella rugulosa, Emericella variecolor, A. sydowii, Aspergillus unguis, A. ustus, A. versicolor, Aspergillus granulosus, and Gigaspora margarita |
| A.nig-S | 12/12 A. niger strains | A. awamori, Aspergillus carbonarius, Aspergillus coreanus, A. foetidus, Aspergillus ibericus, A. phoenicis, Aspergillus wentii, Gliocladium cibottii,a and Verticillium bulbillosuma |
| A.terr-S | 17/20 A. terreus strainsb | None |
| C.alb-S | 32/32 C. albicans strains | None |
| C.dubl-S | 14/14 C. dubliniensis strains | None |
| C.glab-S | 15/15 C. glabrata strains | None |
| C.krus-S | 9/9 C. krusei strains | None |
| C.para-S | 79/79 C. parapsilosis strains | None |
| C.trop-S | 12/12 C. tropicalis strains | None |
Specificity of the Candida-specific biprobes and melting temperature values.
A BLAST search was performed to check the specificity of the Candida-specific biprobes. Each biprobe had 100% sequence similarity to the respective Candida strains in the database. None of those revealed 100% similarity to any other sequence (Table 2). To further prove the specificity, DNA of the fungal isolates described above was tested with each of the Candida species-specific biprobes. Table 1 and Fig. 4 show the Tm values of the biprobe-specific melting peaks. One clinical isolate of C. albicans revealed a biprobe-specific peak Tm of 55°C instead of 66°C. The PCR product of this strain was sequenced and revealed one mismatch in the biprobe-specific annealing site (Fig. 2). Furthermore, C.alb-S showed cross-reactivity with C. dubliniensis (Tm value of 47°C), C.dubl-S showed cross-reactivity with C. albicans (Tm value of 52°C), and C.glab-S showed cross-reactivity with S. cerevisiae (Tm value of 50°C).
Analytical sensitivity.
The lowest Candida or Aspergillus concentration delivering a positive PCR result was 1 CFU per PCR. After DNA extraction, the lowest concentration delivering a positive PCR result was 15 to 30 CFU in 3 ml blood (0.45 to 0.9 CFU per PCR) and 10 CFU in 200 μl CSF or 0.02 g tissue (0.3 CFU per PCR). No change in analytical sensitivity was observed when the five Aspergillus-specific or the six Candida-specific biprobes were used together in one reaction mixture.
Detection of Aspergillus spp. and Candida spp. in clinical samples.
Thirty-one clinical samples derived from patients irrespective of the presence of possible fungal infections were examined by standard cultivation and by real-time PCR (Table 3). In 25 samples (80.6%), fungi were detected by either culture (n = 20), histology (n = 4), or a GM-ELISA (n = 1). For the remaining six samples (19.4%), both culture and histology were negative. When the results of culture were compared with those of real-time PCR, 20 samples (64.5%) (patients 1 to 20) were both culture positive and positive by real-time PCR. In samples 1 to 8, concordant results were obtained by both culture and real-time PCR. In samples 9 to 16, real-time PCR detected not only the fungus grown in culture, but also additional Candida species such as C. albicans and/or C. glabrata. In sample 17, real-time PCR detected C. glabrata, whereas C. lusitaniae was grown by culture. In samples 18 to 20, showing the growth of more than one species, not all of the fungi grown by culture were also detected by real-time PCR. Six samples (19.4%) showed no growth of fungi (samples 21 to 26) but were positive by real-time PCR. Conventional PCR with subsequent sequencing and BLAST analysis performed for the maxillary sinus tissue samples (samples 21 to 24) as described elsewhere (45) confirmed the results of the new real-time PCR assays (data not shown). Furthermore, in samples 21 to 24, the presence of hyphae was proven by histology. In sample 26, infection with Aspergillus was confirmed by GM-ELISA and histology. For the remaining five samples (16.1%), culture, PCR, and histology were concordantly negative.
DISCUSSION
The detection of noncultivable/nonviable cells and circulating free fungal DNA by PCR has been described as an important tool in the early diagnosis of aspergillosis and candidiasis (4). As a rapid diagnosis would improve the survival rate in high-risk patients, we decided to design a real-time PCR approach which enables screening for Aspergillus and Candida infections within a few hours. To our knowledge, no protocol using biprobes to detect and distinguish either Aspergillus or Candida species exists. So far, real-time PCR with biprobes has been restricted to a few studies for the detection of Helicobacter pylori, Campylobacter spp., Mycobacterium tuberculosis, and coagulase-negative staphylococci and for the detection, but not the differentiation, of seven Candida species (10, 11, 28, 36, 43). In this study, the application of the biprobe technology facilitated a rapid screening for and simultaneous differentiation of 11 medically important Aspergillus and Candida species in only two individual PCR mixtures and simultaneously in the same LightCycler run. Biprobes are sequence-specific hybridization probes labeled with the fluorophore Cy5, which is excited by SYBR green I when the probe hybridizes to the target sequence. High specificity of the assay is guaranteed by the right peak Tm, as a probe-specific melting peak with the appropriate Tm can be considered to be a positive result. In both assays, a group-specific and a universal primer were combined with species-specific probes. This enabled a higher degree of specificity than that obtained with genus-specific probes and minimized the risk of false-positive results due to exogenous environmental fungal DNA, such as that of Penicillium, Alternaria, and Saccharomyces species. Nevertheless, one of the drawbacks of the biprobe technology may be the fact that it does not allow for quantification of the pathogen. As a consequence, differentiating between colonization and infection when investigating specimens from sterile body sites may become more difficult. At present, all PCR results of these specimens must be collated with other clinical evidence, such as radiology, culture, histopathology, patient history, and other diagnostic assays.
Our Aspergillus assay includes not only A. fumigatus, but also A. flavus, A. nidulans, A. niger, and A. terreus, which in general extends the diagnostic range of Aspergillus-specific real-time PCR. TaqMan- and HybProbe-based real-time PCR tests for the diagnosis of aspergillosis have so far been targeted to the detection of A. fumigatus only, with the notable exception of an assay described by Costa et al. (7) designed for the detection of A. fumigatus and A. flavus. A PCR-EIA for the detection and differentiation of seven medically important Aspergillus species was designed by de Aguirre et al. (9). Nevertheless, although aspergillosis is mainly due to A. fumigatus, species such as A. flavus, A. terreus, and others are also of clinical importance. The ability to detect and distinguish between the various clinically relevant Aspergillus species is of great diagnostic value, as certain species vary in their resistance to antifungal therapy and are associated with increased virulence and higher mortality. This is mainly the case for A. terreus and A. nidulans, which are frequently resistant to amphotericin B (22, 23). Various PCR targets have been evaluated, but the most commonly used are sequence areas within the ribosomal DNA gene complex (4-8, 19, 20, 26, 30, 32, 34, 35, 39). However, several studies have shown that the ITS1 or ITS2 regions are the most promising targets for refined discrimination between Aspergillus species (9, 17, 33). Our BLAST analyses and alignments of the ITS region revealed that some Aspergillus species are phylogenetically very closely related and show high sequence identity to other fungi, such as Penicillium and Verticillium. In fact, some species cannot be identified to the species level due to identical ITS2 sequences. For example, Aspergillus phoenicis, Aspergillus awamori, Aspergillus foetidus, and Aspergillus tubigensis are molecular siblings of A. niger; Aspergillus oryzae and Aspergillus parasiticus of A. flavus; and Emericella rugulosa and Emericella quadrilineata of A. nidulans. However, this should not be of major concern, as therapy would not be altered substantially for these species. On the other hand, some intraspecies sequence diversity is found in the ITS2 region. Thus, for each Aspergillus species, probes with a 100% intraspecies sequence similarity had to be designed. BLAST analyses and PCR revealed no strain-to-strain variability in the probe binding regions, except for A. terreus. As expected, the intraspecies sequence diversity and the presence of molecular siblings resulted in some potential for cross-reactivity only with very closely related species, as shown in Table 2.
As yet, published Candida-specific real-time PCR assays have been based on species-specific TaqMan probes or hybridization probes (8, 16, 26, 37, 38, 43, 44). Most of these assays facilitated the specific detection of medically important Candida species and were tested on DNA extracted from blood cultures and whole-blood samples. The ability to distinguish between the various clinically relevant Candida species is of eminent importance for guidance on the specific therapy. Although C. krusei is intrinsically resistant to fluconazole and C. glabrata and C. tropicalis may become resistant very quickly during therapy, fluconazole remains the drug of choice to treat invasive candidiasis (41) and the identification of the species is rated as indispensable in critical specimens. For Candida, it is much easier to develop species-specific real-time protocols, since this genus is phylogenetically more diverse than Aspergillus. The Candida-specific assays introduced in the present study were highly specific. Cross-reactions of probes designed for C. albicans, C. dubliniensis, and C. glabrata could easily be detected by a lower (minimum of more than 8°C difference) peak Tm. The ITS2 region showed some intraspecies sequence diversity for several Candida species. However, except for the probe specific for C. albicans, no strain-to-strain variability was observed in the other probe binding regions. Our results are in agreement with those of others suggesting that the ITS2 region is a proper target for the differentiation of Candida species (37).
Both real-time PCR assays proved to be highly sensitive (5 to 10 CFU/ml). For a preliminary clinical evaluation, samples from the respiratory tract and tissue samples were examined by culture and real-time PCR. The combination of a group-specific and a universal primer with five Aspergillus or six Candida species-specific probes in one reaction mixture facilitates rapid screening. However, results showed that the differentiation of species with a peak Tm difference of 1°C is difficult. In addition, with clinical samples, the peak Tm of hybridization probes can vary slightly. This was observed with patients 6 and 19. Here, the C. dubliniensis-specific biprobe was detected at a peak Tm of 63°C instead of 62°C. As a consequence, species confirmation should be performed with the corresponding biprobes whenever the Candida assay reveals a peak Tm between 62°C and 66°C. This is true for C. albicans, C. dubliniensis, C. glabrata, and C. tropicalis. Concerning the Aspergillus assay, A. fumigatus and A. nidulans show the same peak Tm of probes and should also be confirmed in a second PCR.
In the present study, real-time PCR showed mostly a higher sensitivity than culture. Since different forward primers were used for Aspergillus and Candida, simultaneous occurrence of Aspergillus and Candida could be properly determined in the majority of cases. In some samples, PCR even detected a greater number of different Aspergillus and Candida species than culture. In these cases, patients had received antifungal therapy before sampling, which may be a reason for these discrepancies. Furthermore, whenever C. albicans was detected in addition to the cultured Candida (C. glabrata and C. tropicalis) or Aspergillus (A. fumigatus and A. flavus) species, C. albicans might have been overgrown by the other fungi and therefore missed by culture. On the other hand, culture detected a greater number of different species than real-time PCR in three samples, which may be due to an outcompetition of a species present in lower numbers. The higher sensitivity of the real-time PCR was clearly indicated by the results obtained from the fungus balls, all of them being PCR positive and culture negative. Discrepancies in the results of culture and PCR are well known and have already been observed in previous studies (45). In addition, culture of the blood sample from patient 26, with histology-proven aspergillosis, was negative, whereas real-time PCR detected A. flavus. In general, blood culture is considered to be an important tool for the detection of systemic infection, but it has been shown to be positive in less than 50% of patients with chronic disseminated candidiasis (12). Blood cultures of patients suffering from invasive aspergillosis remain negative in almost all cases (18). Also, the sensitivity of cultures from BAL fluid is low. As has been described by Levy et al. (24), Aspergillus spp. are isolated in only 50 to 57% of all cases.
However, it has to be considered that the newly developed assays cover the range of the most important, but not every, Aspergillus and Candida species. This is illustrated by a sample for which real-time PCR did not detect C. lusitaniae, since the Candida-specific assay does not include a biprobe for this species. PCR detected C. glabrata instead. A cross-reaction of C. lusitaniae with C. glabrata can be excluded, as primer Cand-F shows 2 mismatches at the 3′ end with C. lusitaniae, which would most probably prevent the amplification of C. lusitaniae in a clinical sample. Furthermore, the biprobe C.glab-S reveals 10 mismatches with the ITS2 region of C. lusitaniae.
In summary, this is the first real-time PCR assay which allows the simultaneous detection and identification of various Candida and Aspergillus species. The results of the analytical and clinical evaluations show that both assays are highly sensitive and can be used in clinical laboratories as simple screening tests for the most commonly encountered Aspergillus and Candida species. As our results have been quite promising, it is planned to evaluate the clinical impact of these assays in combination with other tests, such as the detection of GM, in a prospective study which will start in the near future.
Acknowledgments
We thank A. Makristathis for helpful discussion on the manuscript.
Footnotes
Published ahead of print on 24 January 2007.
REFERENCES
- 1.Ajello, L., and R. J. Hay (ed.). 1998. Medical mycology, p. 1-711. Edward Arnold, London, United Kingdom.
- 2.Ascioglu, S., J. H. Rex, B. de Pauw, J. E. Bennett, J. Bille, F. Crokaert, D. W. Denning, J. P. Donnelly, J. E. Edwards, Z. Erjavec, D. Fiere, O. Lortholary, J. Maertens, J. F. Meis, T. F. Patterson, J. Ritter, D. Selleslag, P. M. Shah, D. A. Stevens, and T. J. Walsh. 2002. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34:7-14. [DOI] [PubMed] [Google Scholar]
- 3.Beck-Sague, C. M., W. R. Jarvis, et al. 1993. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980-1990. J. Infect. Dis. 167:1247-1251. [DOI] [PubMed] [Google Scholar]
- 4.Bretagne, S., and J.-M. Costa. 2005. Towards a molecular diagnosis of invasive aspergillosis and disseminated candidosis. FEMS Immunol. Med. Microbiol. 45:361-368. [DOI] [PubMed] [Google Scholar]
- 5.Challier, S., S. Boyer, E. Abachin, and P. Berche. 2004. Development of a serum-based TaqMan real-time PCR assay for diagnosis of invasive aspergillosis. J. Clin. Microbiol. 42:844-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa, C., D. Vidaud, M. Olivi, E. Bart-Delabesse, M. Vidaud, and S. Bretagne. 2001. Development of two real-time quantitative TaqMan PCR assays to detect circulating Aspergillus fumigatus DNA in serum. J. Microbiol. Methods 44:263-269. [DOI] [PubMed] [Google Scholar]
- 7.Costa, C., J. M. Costa, C. Desterke, F. Botterel, C. Cordonnier, and S. Bretagne. 2002. Real-time PCR coupled with automated DNA extraction and detection of galactomannan antigen in serum by enzyme-linked immunosorbent assay for diagnosis of invasive aspergillosis. J. Clin. Microbiol. 40:2224-2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cruz-Perez, P., M. P. Buttner, and L. D. Stetzenbach. 2001. Detection and quantitation of Aspergillus fumigatus in pure culture using polymerase chain reaction. Mol. Cell. Probes 15:81-88. [DOI] [PubMed] [Google Scholar]
- 9.de Aguirre, L., S. F. Hurst, J. S. Choi, J. H. Shin, H. P. Hinrikson, and C. J. Morrison. 2004. Rapid differentiation of Aspergillus species from other medically important opportunistic molds and yeasts by PCR-enzyme immunoassay. J. Clin. Microbiol. 42:3495-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards, K. J., M. E. Kaufmann, and N. A. Saunders. 2001. Rapid and accurate identification of coagulase-negative staphylococci by real-time PCR. J. Clin. Microbiol. 39:3047-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards, K. J., L. A. Metherell, M. Yates, and N. A. Saunders. 2001. Detection of rpoB mutations in Mycobacterium tuberculosis by biprobe analysis. J. Clin. Microbiol. 39:3350-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Einsele, H., H. Hebart, G. Roller, J. Loffler, I. Rothenhofer, C. A. Muller, R. A. Bowden, J. van Burik, D. Engelhard, L. Kanz, and U. Schumacher. 1997. Detection and identification of fungal pathogens in blood by using molecular probes. J. Clin. Microbiol. 35:1353-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espy, M. J., J. R. Uhl, L. M. Sloan, S. P. Buckwalter, M. F. Jones, E. A. Vetter, J. D. Yao, N. L. Wengenack, J. E. Rosenblatt, F. R. Cockerill III, and T. F. Smith. 2006. Real-time PCR in clinical microbiology: applications for routine laboratory testing. Clin. Microbiol. Rev. 19:165-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferns, R. B. 2006. Evaluation of the role of real-time PCR in the diagnosis of invasive aspergillosis. Leuk. Lymphoma 47:15-20. [DOI] [PubMed] [Google Scholar]
- 15.Fidel, P. L., J. A. Vazquez, and J. D. Sobel. 1999. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin. Microbiol. Rev. 12:80-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guiver, M., K. Levi, and B. A. Oppenheim. 2001. Rapid identification of Candida species by TaqMan PCR. J. Clin. Pathol. 54:362-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hinrikson, H. P., S. F. Hurst, T. J. Lott, D. W. Warnock, and C. J. Morrison. 2005. Assessment of ribosomal large-subunit D1-D2, internal transcribed spacer 1, and internal transcribed spacer 2 regions as targets for molecular identification of medically important Aspergillus species. J. Clin. Microbiol. 43:2092-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hopfer, R. L. 1997. Contemporary techniques for molecular diagnoses of mycoses. Clin. Microbiol. Newsl. 19:169-173. [Google Scholar]
- 19.Imhof, A., C. Schaer, G. Schoedon, D. J. Schaer, R. B. Walter, A. Schaffner, and M. Schneemann. 2003. Rapid detection of pathogenic fungi from clinical specimens using LightCycler real-time fluorescence PCR. Eur. J. Clin. Microbiol. Infect. Dis. 22:558-560. [DOI] [PubMed] [Google Scholar]
- 20.Kami, M., T. Fukui, S. Ogawa, Y. Kazuyama, U. Machida, Y. Tanaka, Y. Kanda, T. Kashima, Y. Yamazaki, T. Hamaki, S. Mori, H. Akiyama, Y. Mutou, H. Sakamaki, K. Osumi, S. Kimura, and H. Hirai. 2001. Use of real-time PCR on blood samples for diagnosis of invasive aspergillosis. Clin. Infect. Dis. 33:1504-1512. [DOI] [PubMed] [Google Scholar]
- 21.Klingspor, L., and S. Jalal. 2006. Molecular detection and identification of Candida and Aspergillus spp. from clinical samples using real-time PCR. Clin. Microbiol. Infect. 12:745-753. [DOI] [PubMed] [Google Scholar]
- 22.Kontoyiannis, D. P., R. E. Lewis, G. S. May, N. Osherov, and M. G. Rinaldi. 2002. Aspergillus nidulans is frequently resistant to amphotericin B. Mycoses 45:406-407. [DOI] [PubMed] [Google Scholar]
- 23.Lass-Flörl, C., G. Kofler, G. Kropshofer, J. Hermans, A. Kreczy, M. P. Dierich, and D. Niederwieser. 1998. In-vitro testing of susceptibility to amphotericin B is a reliable predictor of clinical outcome in invasive aspergillosis. J. Antimicrob. Chemother. 42:497-502. [DOI] [PubMed] [Google Scholar]
- 24.Levy, H., D. A. Horak, B. R. Tegtmeier, S. B. Yokota, and S. J. Forman. 1992. The value of bronchoalveolar lavage and bronchial washings in the diagnosis of invasive pulmonary aspergillosis. Respir. Med. 86:243-248. [DOI] [PubMed] [Google Scholar]
- 25.Loeffler, J., H. Hebart, U. Schumacher, H. Reitze, and H. Einsele. 1997. Comparison of different methods for extraction of DNA of fungal pathogens from cultures and blood. J. Clin. Microbiol. 35:3311-3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loeffler, J., N. Henke, H. Hebart, D. Schmidt, L. Hagmeyer, U. Schumacher, and H. Einsele. 2000. Quantification of fungal DNA by using fluorescence resonance energy transfer and the LightCycler system. J. Clin. Microbiol. 38:586-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marr, K. A., R. Carter, F. Crippa, A. Wald, and L. Corey. 2002. Epidemiology and outcome of mould infections in hematopoetic stem cell transplant recipients. Clin. Infect. Dis. 34:909-917. [DOI] [PubMed] [Google Scholar]
- 28.Menard, A., F. Dachet, V. Prouzet-Mauleon, M. Oleastro, and F. Megraud. 2005. Development of a real-time fluorescence resonance energy transfer PCR to identify the main pathogenic Campylobacter spp. Clin. Microbiol. Infect. 11:281-287. [DOI] [PubMed] [Google Scholar]
- 29.Ostrosky-Zeichner, L., J. H. Rex, P. G. Pappas, R. J. Hamill, R. A. Larsen, H. W. Horowitz, W. G. Powderly, N. Hyslop, C. A. Kauffman, J. Cleary, J. E. Mangino, and J. Lee. 2003. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob. Agents Chemother. 47:3149-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Sullivan, C. E., M. Kasai, A. Francesconi, V. Petraitis, R. Petraitiene, A. M. Kelaher, A. A. Sarafandi, and T. J. Walsh. 2003. Development and validation of a quantitative real-time PCR assay using fluorescence resonance energy transfer technology for detection of Aspergillus fumigatus in experimental invasive pulmonary aspergillosis. J. Clin. Microbiol. 41:5676-5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfaller, M. A., D. J. Diekema, S. A. Messer, L. Boyken, and R. J. Hollis. 2003. Activities of fluconazole and voriconazole against 1,586 recent clinical isolates of Candida species determined by broth microdilution, disk diffusion, and Etest methods: report from the ARTEMIS Global Antifungal Susceptibility Program, 2001. J. Clin. Microbiol. 41:1440-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pryce, T. M., I. D. Kay, S. Palladino, and C. H. Heath. 2003. Real-time automated polymerase chain reaction (PCR) to detect Candida albicans and Aspergillus fumigatus DNA in whole blood from high-risk patients. Diagn. Microbiol. Infect. Dis. 47:487-496. [DOI] [PubMed] [Google Scholar]
- 33.Rakeman, J. L., U. Bui, K. Lafe, Y. C. Chen, R. J. Honeycutt, and B. T. Cookson. 2005. Multilocus DNA sequence comparisons rapidly identify pathogenic molds. J. Clin. Microbiol. 43:3324-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rantakokko-Jalava, K., S. Laaksonen, J. Issakainen, J. Vauras, J. Nikoskelainen, M. K. Viljanen, and J. Salonen. 2003. Semiquantitative detection by real-time PCR of Aspergillus fumigatus in bronchoalveolar lavage fluids and tissue biopsy specimens from patients with invasive aspergillosis. J. Clin. Microbiol. 41:4304-4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanguinetti, M., B. Posteraro, L. Pagano, G. Pagliari, L. Fianchi, L. Mele, M. La Sorda, A. Franco, and G. Fadda. 2003. Comparison of real-time PCR, conventional PCR, and galactomannan antigen detection by enzyme-linked immunosorbent assay using bronchoalveolar lavage fluid samples from hematology patients for diagnosis of invasive pulmonary aspergillosis. J. Clin. Microbiol. 41:3922-3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schabereiter-Gurtner, C., A. M. Hirschl, B. Dragosics, P. Hufnagl, S. Puz, S. Kovách, M. Rotter, and A. Makristathis. 2004. Novel real-time PCR assay for detection of Helicobacter pylori infection and simultaneous clarithromycin susceptibility testing in stool and biopsy specimens. J. Clin. Microbiol. 42:4512-4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selvarangan, R., U. Bui, A. P. Limaye, and B. T. Cookson. 2003. Rapid identification of commonly encountered Candida species directly from blood culture bottles. J. Clin. Microbiol. 41:5660-5664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin, J. H., F. S. Nolte, B. P. Holloway, and C. J. Morrison. 1999. Rapid identification of up to three Candida species in a single reaction tube by a 5′ exonuclease assay using fluorescent DNA probes. J. Clin. Microbiol. 37:165-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spiess, B., D. Buchheidt, C. Baust, H. Skladny, W. Seifarth, U. Zeilfelder, C. Leib-Mosch, H. Morz, and R. Hehlmann. 2003. Development of a LightCycler PCR assay for detection and quantification of Aspergillus fumigatus DNA in clinical samples from neutropenic patients. J. Clin. Microbiol. 41:1811-1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sullivan, D., K. Haynes, J. Bille, P. Boerlin, L. Rodero, S. Lloyd, M. Henman, and D. Coleman. 1997. Widespread geographic distribution of oral Candida dubliniensis strains in human immunodeficiency virus-infected individuals. J. Clin. Microbiol. 35:960-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vanden Bossche, H., F. Dromer, I. Improvisi, M. Lozano-Chiu, J. H. Rex, and D. Sanglard. 1998. Antifungal drug resistance in pathogenic fungi. Med. Mycol. 36:19-28. [PubMed] [Google Scholar]
- 42.Verweij, P. E., and J. F. Meis. 2000. Microbiological diagnosis of invasive fungal infections in transplant recipients. Transplant Infect. Dis. 2:80-87. [DOI] [PubMed] [Google Scholar]
- 43.White, P. L., A. Shetty, and R. A. Barnes. 2003. Detection of seven Candida species using the Light-Cycler system. J. Med. Microbiol. 52:229-238. [DOI] [PubMed] [Google Scholar]
- 44.White, P. L., D. W. Williams, T. Kuriyama, S. A. Samad, M. A. Lewis, and R. A. Barnes. 2004. Detection of Candida in concentrated oral rinse cultures by real-time PCR. J. Clin. Microbiol. 42:2101-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willinger, B., A. Obradovic, B. Selitsch, J. Beck-Mannagetta, W. Buzina, H. Braun, P. Apfalter, A. M. Hirschl, A. Makristathis, and M. Rotter. 2003. Detection and identification of fungi from fungus balls of the maxillary sinus by molecular techniques. J. Clin. Microbiol. 41:581-585. [DOI] [PMC free article] [PubMed] [Google Scholar]