Abstract
Photoreceptor outer segment (OS) renewal requires a series of tightly regulated membrane fusion events which are mediated by a fusion complex containing protein and lipid components. The best characterized of these components, is a unique photoreceptor specific tetraspanin, peripherin/rds (P/rds, a.k.a., peripherin-2, Rds and Prph). In these studies we investigated the role of peripherin’s non-glycosylated homolog, ROM-1, in OS fusion using a COS cell heterologous expression system and a well characterized cell free fusion assay system. Membranes isolated from COS-7 cells transfected with either FLAG-tagged P/rds or HA-tagged ROM-1 or both proteins were assayed for their ability to merge with fluorescently labeled OS plasma membrane (PM). Such membrane merger is one measure of membrane fusogenicity. The highest percent fusion was observed when the proteins were co-expressed. Furthermore detailed analysis of the fusion kinetics between fluorescently labeled PM and proteo-liposomes containing either, pure P/rds, pure ROM-1 or the ROM-1-P/rds complex clearly demonstrated that optimal fusion requires an ROM-P/rds1 complex. Proteo-liposomes composed of ROM-1 alone were not fusogenic. Peptide competition studies suggest that optimization of fusion may be due to the formation of a fusion competent peripherin/rds C-terminus in the presence of ROM-1. These studies provide further support for the hypothesis that a P/rds dependent membrane fusion complex is involved in photoreceptor renewal processes.
Keywords: retina, photoreceptors, peripherin-2, P/rds, ROM-1, outer segment renewal
Introduction
Photoreceptor cell structure is maintained through the coordinated processes of disk morphogenesis and disk shedding. Both of these processes requires tightly regulated membrane fusion events, in the delivery of new membraneous material from the IS (Deretic and Papermaster 1991; Deretic and Papermaster 1993; Deretic and Papermaster 1995; Chuang, Vega et al. 2004), for disk closure during morphogenesis (Steinberg, Fisher et al. 1980; Boesze-Battaglia and Goldberg 2002) and in disk packet formation and shedding (Boesze-Battaglia and Goldberg 2002; Chen, Yunhai et al. 2002). Previous studies in our laboratory have suggested that disks at the apical tip of the OS fuse spontaneously to delineate a packet of disks (Boesze-Battaglia 1997) and that the tetraspanin protein, peripherin/rds (P/rds a.k.a. Prph, Rds and peripherin-2) participates in this membrane fusion process in vitro (Boesze-Battaglia 1997; Boesze-Battaglia, Kong et al. 1997; Boesze-Battaglia, Lamba et al. 1998). Deletion of a region including the amphiphilic fusion peptide domain of P/rds in transgenic Xenous laevis resulted in the mis-localization of the mutated P/rds (Tam, Moritz et al. 2002; Tam, Moritz et al. 2004), supporting a key functional role for this domain. Moreover the multi-functionality of P/rds C-terminus is further supported by a newly generated transgenic mouse, in which a loss of P/rds fusion function transgene expressed on an rds heterozygote background failed to rescue the rds +/− phenotype and moreover resulted in altered phagocytosis (Goldberg, Ritter et al. 2006).
A murine model of retinitis pigmentosa (RP) in which a 10 kb insertion of exogeneous DNA results in an RDS null allele provides further support for P/rds as a component of a fusion complex (Travis, Brennan et al. 1989; Connell, Bascom et al. 1991; Cheng, Peachey et al. 1997). Mice homozygous for the RDS mutation are absent of OS and show almost complete deterioration of photoreceptor cell layer by 12 months of age (Sanyal and Jansen 1981; Sanyal and Hawkins 1988). P/rds heterozygotes exhibit irregular OS, altered disk shedding and phagocytosis (Hawkins, Jansen et al. 1985). The dominant negative phenotype of the 307-del mouse model of RP, in which the C-terminal domain of P/rds is elongated due to the deletion of a codon 307, exhibits a more rapid retinopathy than the rds −/−. This phenotype led the authors to conclude that the C-terminus of P/rds contains a unique functional domain that contributes to the degenerative process (McNally, Kenna et al. 2002). Digenic RP (Kajiwara, Berson et al. 1994) suggests that although P/rds and ROM-1 cooperate to generate healthy photoreceptors, they are not functionally equivalent and ROM-1 likely plays a subsidiary role. Biochemical studies showed that in digenic RP, ROM-1 homotetramers do not compensate for P/rds–ROM-1 hetero-tetramers (Goldberg and Molday 1996; Loewen, Moritz et al. 2001). Lastly, data from a chimeric mouse line expressing the D-2 loop of P/rds in the context of ROM-1 suggest that functional efficacy is not restricted to the D-2 loop (Kedzierski, Weng et al. 1999).
Although ROM-1 forms a hetero-tetrameric complex with P/rds the precise functional role of this complex and of ROM-1 specifically is largely unknown. ROM-1 knockout mice, for example, show a relatively mild phenotype; dysmorphic OS with disks that appear to be unusually large, with P/rds localization to the disk rims appearing relatively normal (Clarke, Goldberg et al. 2000). This phenotype suggests that ROM-1 plays an accessory role in P/rds dependent processes. These processes include the maintenance of OS structure through alignment of newly forming disks (Goldberg and Molday 1996; Goldberg and Molday 1996; Tam, Moritz et al. 2004) targeting of P/rds to the OS through a C-terminal signal sequence (Tam, Moritz et al. 2001; Tam, Moritz et al. 2002), interactions with GARP linking the disk rim to the cGMP gated channel (Körschen, Beyermann et al. 1999; Poetsch, Molday et al. 2001) and participation in membrane fusion (Boesze-Battaglia, Lamba et al. 1998). Little if any information is available regarding the role of ROM-1 in any of these processes. Work in our laboratory has focused on understanding how membrane fusion processes coordinate to main healthy photoreceptors. In this study we investigated if ROM-1 plays a role in photoreceptor membrane fusion using a COS cell heterologous expression system and a well characterized cell free assay system. Our results suggest that although ROM-1 is not inherently fusogenic it is likely an accessory protein participating in the formation of a fusion complex.
Materials and Methods
Plasmid constructs
Procedures for the isolation and cloning of bovine FLAG-tagged peripherin/rds (FLAG- P/rds) and hemaglutininin-tagged ROM-1, ( HA-ROM-1) have previously been described (Muller-Weeks, Boesze-Battaglia et al. 2002). Primer design was based on sequences reported by (Connell and Molday 1990) and (Moritz and Molday 1996).
Cell culture and transfection
SV40 transformed kidney fibroblast cells from the monkey, Cercopithecus aethiops (COS-7), were grown in Dulbecco’s Modified Essential Media (DMEM) as per ATCC (American Type Culture Collection) protocols. Cells were routinely split 1:3 every third or fourth day and transfected using Lipofectamine PLUS reagent (GIBCO/BRL). The day before transfection, cells were seeded according to the size of the culture vessel used; 1X105 cells/ well in a six well plate, 1X106 cells/ 10 cm dish, or 3X106 cells/ 15 cm dish. Cells were harvested 48 hours post-transfection.
Purification of ROM-1 from bovine retinas
ROM-1 was purified using a strategy originally developed for the purification of P/rds that relied on a combination of Concanavalin-A Sepharose affinity chromatography and chromatofocusing (Boesze-Battaglia, Kong et al. 1997). Briefly, ROS disk membranes were prepared from frozen, dark-adapted retinas (J. Lawson Inc.) using Ficoll flotation (Smith, Stubbs et al. 1975). The isolated ROS disk membranes were washed in hypotonic buffer (5 mM Hepes and 1 mM EDTA at pH 7.8) prior to Con-A chromatography. All manipulations of ROS membranes were performed under dim red light, and buffers purged with argon to reduce lipid oxidation. Con-A chromatography was carried out as described (Litman 1982). The hypotonically washed ROS disk membranes were washed, resuspended in Con-A standard buffer and solubilized in 30 mM OG. Fractions were monitored at 280 nm, and peak fractions corresponding to the flow-through (unbound) peak were pooled. The unbound fraction, enriched in P/RDS and ROM-1 was dialyzed for 48 hrs with four changes of 10 mM Hepes, 100 mM NaCl to form proteo-liposomes, designated in this study as rim specific vesicles (RSV). For ROM-1 purification the unbound fractions were concentrated by Amicon ultrafiltration (model 8050) using a YM-30 filter, to 1/10 the original volume. The concentrated samples were dialyzed overnight against 0.025 M imidazole hydrochloride, (pH 7.4), thereby reducing the OG concentration from 146 mM to less than 40 mM.
ROM-1 was separated from P/rds by chromatofocusing as described in detail (Boesze-Battaglia, Kong et al. 1997). Mono Q HR 5/5 columns, PBE-94 and Polybuffer were purchased from Pharmacia. Briefly, PBE-94 column material was equilibrated with 0.025 M imidazole hydrochloride and 10 mM OG (pH 7.4) until a stable pH was established. Prior to the loading of the column, 1 mL of start buffer was added so that the sample proteins were not exposed to extremes of pH. Proteins were eluted with the appropriate dilution of Polybuffer-74 and 10 mM OG as described below. Eluate absorbance was monitored at 280 nm. Routinely, 0.750–1.2 mL fractions were collected. The pH of every second fraction was recorded immediately. For pH range 7-4, PBE-94 was used in a 5–7 mL bed volume. Start buffer was 0.025 M imidazole hydrochloride and 10 mM OG at pH 7.4; eluate was a 1:8 dilution of polybuffer-74 hydrochloride at pH 4.0 and 10 mM OG. The chromatofocused fractions were assayed for protein (Bio-Rad) and the fractions pooled and concentrated to 0.5 mL using Centricon-30 concentrators (Amicon) prior to SDS-PAGE and Western Blot analysis. ROM-1 was routinely recovered in fractions #75-80, corresponding to a pH =5.40 to 5.32.
Western blot and immunoprecipitation analysis
Protein expression was assessed by western blotting as described by Towbin et al (Towbin, Staehelin et al. 1979). For these experiments, COS cells (1 X 106) were seeded in 10 cm culture dishes and transfected with 4 μg of the indicated constructs, cell extracts prepared and subjected to 12% SDS-PAGE under reducing conditions prior to Immunoblotting. Western blots were probed with anti-FLAG M5 monoclonal antibody at 10 μg/ml (Sigma) or anti-HA monoclonal antibody at a 1:1000 dilution (BabCo, Richmond, CA) followed by 1:1000 dilution of alkaline phosphatase-conjugated goat-α-mouse 2° antibody (1:3000 dilution). Bands were visualized using a Sigma Fast BCIP/NBT (5-Bromo-4-Chloro-3-Indolyl phosphate/ Nitro blue tetrazolium) alkaline phosphatase substrate. Digital analysis of blots was performed using Kodak Image Station 440CF.
For co-immunoprecipitation studies, COS cells (1 X 106) were seeded in 10 cm dishes and co-transfected with 2 μg of either FLAG-P/rds or HA-ROM-1. Forty-eight hours post transfection cells were harvested and extracts prepared in 100 μl lysis buffer containing 0.1% NP-40. 50 μl of each extract was precipitated in a 1 ml volume overnight on ice at 4°C with 10 μg/ ml anti-FLAG M5 monoclonal antibody or with a 1:1000 dilution of anti-HA monoclonal antibody. The following day, specific complexes were recovered by adding 150 μl of protein A sepharose (10% W/V) followed by 5 washes with 1 ml of the 0.1% NP-40 lysis buffer. 300 μl of 1 mg/ml MOP-C21 purified immunoglobulin (Sigma) bound to 400 μl of protein A-sepharose beads was used as a negative control. Prior to separation by 12.6% SDS-PAGE, 30 μl of 2X SDS loading buffer and 1μl BME were added to the protein A sepharose. The mixture was heated to 80°C for 10 minutes and aliquots used for Western blot as described.
Immunohistochemistry
For immunohistochemistry, COS-7 cells (1 X 105) were seeded on coverslips in six well dishes and transfected or co-transfected with the indicated constructs. Forty-eight hours post transfection, cells were washed in PBS (phosphate buffered saline), fixed, permeabilized in methanol/acetone (1:1) and air dryed. Coverslips were incubated overnight with 10 μg/ ml anti-FLAG M5 monoclonal antibody and/or a 1:1000 dilution of anti-HA polyclonal antibody, washed and incubated at a 1:1000 dilution of FITC-conjugated goat-α-mouse secondary antibody and a 1:500 dilution of CY3-conjugated goat-α-rabbit secondary antibody for one hour in the dark. Subsequently, the cells were washed again with PBS and viewed through a Ziess Axioscope fluorescence microscope equipped with an FITC-filter, a Rhodamine filter, and a dual FITC/Rhodamine filter. Analysis of fluorescent probe co-localization was performed using image analysis software Metamorph; (Universal Imaging Corporation; Downingtown, PA).
Purification of bovine rod outer segment disk membranes, plasma membrane vesicles and RSVs
Bovine ROS disk and plasma membranes were prepared for fusion assays as described in detail (Boesze-Battaglia, Albert et al. 1992). The plasma membrane forms vesicles as the OS is broken and reseals (Boesze-Battaglia, Albert et al. 1992).When the plasma membrane reseals it forms both inside out and outside out vesicles (Boesze-Battaglia 1997). The orientation reflects the extra cellular plasma membrane surface or the intra-diskal surface Vesicles enriched in P/rds and rom-1 were prepared essentially as described previously and are designated rim specific vesicles (RSV) (Boesze-Battaglia, Kong et al. 1997). Rim specific vesicles (RSV’s) were prepared from the dialyzed lipid rich fraction of ROS–disk Con A chromatography by freeze/thawing as described above. RSV’s were diluted 1:10 in 2%w/v BSA, 10mM Tris pH 7.5, 150mM NaCl and incubated on ice for 30 minutes. RSV’s were centrifuged at 65,000 rpm for 30 minutes and the resulting pellet resuspended in 8 mls of 1%w/v BSA, 10mM Tris pH 7.5, 150mM NaCl along with 10 μl of anti-P/rds mAb antibody 2B6. Vesicles were incubated at room temperature for 30 minutes on an orbital rocker. The 2B6 labeled RSV’s were then centrifuged at 65,000 rpm and the pellet washed twice in 40mM Tris HCl, 10mM Tris Base pH 8.0, 150mM NaCl. The final pellet was resuspended in 8 mls of antibody binding buffer and 8ul of Goat Anti-mouse IgG-FITC conjugate–(Gibco BRL) was added. The vesicles were incubated at room temperature for 30 minutes on an orbital rocker, centrifuged and washed as in the previous steps. The final pellet was resuspended in phosphate buffered saline to a final volume of 1 ml.
COS cell intracellular membrane isolation
Intracellular membranes from transfected COS cells were isolated as described by (Oprian 1993) with slight modification (Muller-Weeks, Boesze-Battaglia et al. 2002). COS cells seeded in 15 cm culture dishes (3 X 107 cells/ dish) were transfected and harvested 48hr post transfection, by scraping in 10 ml of 10 mM Hepes (pH 7.4). Cells were resuspended in 600 μl Tris-MgCl2 buffer (10 mM Tris, 2 mM MgCl2) and lysed by passage through a 26 gauge needle twice. COS cell lysates were layered on 3.8 ml of 37% W/V sucrose and centrifuged at 18,000 rpm for 20 minutes at 10°C (SW60 rotor, Beckman instruments). Intracellular membranes were recovered in 500 μl and an equal volume of Tris-MgCl2 buffer was added to dilute the sucrose.
Fluorescent labeling of purified ROS disk and plasma membrane
COS-7 cell intracellular membrane and ROS disk membrane phospholipid concentration was determined as described by Bartlett (Bartlett 1959) and modified by (Litman 1973). Aliquots of COS cell membranes were labeled with either Octadecylrhodamine B chloride (R18) or 5-(N-octadecanoyl) aminofluorescein (F18, Molecular Probes, Inc., Junction City, OR) at 1 mole% relative to phospholipid for FRET assays. In contrast, for dequenching assays, plasma membrane vesicles were labeled with the R18 at 3 mole % relative to the ROS total phospholipid as described (Boesze-Battaglia, Kong et al. 1997; Boesze-Battaglia, Lamba et al. 1998). Labeled membranes were separated from unincorporated probe by size exclusion chromatography through a Sephadex G-50 column (Boesze-Battaglia, Kong et al. 1997; Boesze-Battaglia, Lamba et al. 1998).
Resonance energy transfer fusion assays
Membrane fusion was measured using fluorescence resonance energy transfer (FRET) fusion assays (Partearroyo, Cabezon et al. 1994; Boesze-Battaglia 2000). In this cell free assay system, one fusion partner, COS membranes expressing FLAG-P/RDS or ROS disk membranes were labeled with F18, while the other fusion partner, ROS plasma membrane, was labeled with R18. These specific fluorochromes were chosen because the emission spectrum of one (F18) overlaps the excitation spectrum of the other (R18). The close association of the probes as occurs during fusion allows energy transfer from F18 (λex=460 nm) to R18, thereby indirectly exciting R18, resulting in an increase in R18 fluorescence λem=592nm and consequently, a decrease in F18 emission. All fusion assays were carried out at room temperature under dim light. Fusion was followed on a Perkin-Elmer LS 55B spectrofluorometer (Gaithersburg, MD) equipped with a 96 well plate reader at room temperature. Fusion was initiated with the addition of R18PM membrane to F18-COS cell membrane or F18-labeled ROS disk membrane already present in the well. Optimal fusion was detected using 100 μl of membrane suspension (70 μl plasma membrane and 30 μl COS membrane). For fusion assays including the inhibitor peptide PP5, 10 μg of the inhibitor was added to the F18-labeled membrane sample in the well prior to the addition of R18-labeled ROS plasma membrane. For all fusion assays, fluorescence intensity was measured at an λex=460 nm (F18 excitation) and simultaneously at λem =524 nm (F18 emission) and λem =592 nm (R18 emission) over a 2 minute period. The fusion was calculated as the change in R18 intensity over time. The change in R18 intensity at a given time was calculated as follows:
where I524 and I592 are the fluorescence intensities at 524 and 592 nm, respectively. The subscripts T and I represent a given time point and the initial time point of each sample, respectively. Values for I592 were corrected by subtracting the F18 contribution to the R18 λem = 592 nm. This value was determined by performing a scan of the fluorescence λem = 592 and 524 nm when F18 was the only probe present in the well. The % change in R18 was determined by multiplying each ΔR value by 100. PCI-Neo transfected COS cells were used as the negative control. Background % change in R18 was calculated for the pCI-Neo negative control and subtracted from the P/rds COS-7 cell intracellular membrane results. The maximum % change in R18 intensity achieved for R18-labeled plasma membrane and F18-labeled ROS disk fusion was set at 100% maximum fusion. This value was used to calculate the % maximum change in R18 for each of the other fusion assay results.
R18 lipid mixing assay
Fusion assays characterizing R18-labeled plasma membrane-disk membrane or RSV fusion were performed exactly as described (Boesze-Battaglia, Albert et al. 1992). In some experiments, disk membranes were pretreated with either, anti-P/rds mAb 2B6, anti-ROM-1 antibody, mAb 1D5, or various peptides for 10 min in the dark at 37oC. Bradykinin (Sigma) was used as a nonspecific control peptide in these studies. All peptides were added at a concentration equivalent to 10 μmol of peptide per mole P/RDS. Fluorescence was measured on Perkin-Elmer LS-55B spectrofluorometer at a λex = 560 nm and λem = 586 nm. Fusion was initiated with the addition of R18-labeled plasma membrane vesicles (R18-PM) to various target membranes; disks, RSV or proteo-liposomes. The increase in R18 fluorescence due to the dilution of the probe in the target membrane was monitored continuously and increased linearly with probe dilution. Fluorescence intensity obtained without the addition of plasma membrane was taken as a baseline, fluorescence at infinite probe dilution (100% fluorescence) was determined with the addition of 100 μL of 10% Triton X-100 to the membrane mixture. In these experiments, the increase in fluorescence was recorded for 10 min. during which time the fluorescence reached a plateau. The change in fluorescence over the first 5 min was used to calculate the initial rate of fusion. Fusion kinetics was determined as described (Hoekstra, Boer et al. 1984; Hoekstra and Klappe 1986; Boesze-Battaglia, Lamba et al. 1998; Boesze-Battaglia, Stefano et al. 2000), with the lag-time corresponding to time before an increase in R18 fluorescence is observed indicative of membrane mixing and fusion (Boesze-Battaglia 2000). This assay has been shown to be sensitive (without artifacts) to the fusion of disk membranes with large unilamellar vesicles (LUVs) of phosphatidylethanolamine and disk lipid vesicles as well as fusion between plasma membrane and disk membranes (Boesze-Battaglia, Fliesler et al. 1992; Boesze-Battaglia and Yeagle 1992; Boesze-Battaglia 1997; Boesze-Battaglia, Kong et al. 1997). Control LUVs were prepared form disk membrane extracts or COS-7 cell membrane extracts as described previously (Boesze-Battaglia, Fliesler et al. 1992; Boesze-Battaglia and Yeagle 1992; Boesze-Battaglia 1997; Boesze-Battaglia, Kong et al. 1997). The following peptides were used in competition studies ROM-1 C-terminal peptide, RM-1, 299VIDGEGEAQGYLFPG314, P/rds C-terminal amphiphilic peptide PP-5 311VPETWKAFLESVKKL325 (Boesze-Battaglia, Kong et al. 1997) and a peptide corresponding to the ROM-1 binding site on P/rds, RM-2 165CCGNNGFRDWFEIQW182 (Ding, Stricker et al. 2005).
Results
ROM-1 forms a hetero-tetrameric complex with P/rds both in vivo and in heterologous cell expression systems (Goldberg, Moritz et al. 1995; Goldberg and Molday 1996; Muller-Weeks, Boesze-Battaglia et al. 2002). Even though the domain involved in tetramerization has been mapped to Cys165-Asn182 of P/rds the precise role of ROM-1 in P/rds dependent function has remained elusive. In this series of studies we tested the hypothesis that ROM-1 plays an accessory role in photoreceptor specific membrane fusion processes. We used a heterologous cell expression system in which COS-7 cells were transfected with either HA-ROM-1 or FLAG- P/rds (Muller-Weeks, Boesze-Battaglia et al. 2002) or co-transfected in addition to a cell free assay system consisting of proteo-liposome target membranes (Boesze-Battaglia, Kong et al. 1997; Boesze-Battaglia, Lamba et al. 1998).
COS-7 cells were co-transfected with HA-ROM-1 and FLAG- P/rds as described previously (Muller-Weeks, Boesze-Battaglia et al. 2002). Quantitative immuno-blots using a combination of both anti-FLAG and anti-HA antibodies as a well as anti- P/rds (mAb 2B6) and anti-ROM-1 (mAb 1D5) suggest equal levels of protein expression (Fig. 1A). As shown in Fig. 1B, HA-ROM-1 and FLAG P/rds localize to intracellular membranes consistent with previous studies (Goldberg, Moritz et al. 1995). Co-localization was quantitated using Metamorph analysis with 84 +/− 7.9% of FLAG- P/rds co-localizing with HA-ROM-1 and 79 +/− 6.5 % of HA-ROM-1 co-localizing with FLAG- P/rds (Fig. 1C) based on average pixel intensity. Lastly, the formation of ROM-1-P/rds complexes in COS-7 cell membrane extracts was confirmed by co-immunoprecipitation. Immunoprecipitates with anti-FLAG antibody pulled down HA-ROM-1 and in co-immunoprecipitations, anti-HA antibody immunoprecipitated a HA-ROM-1-FLAG-P/rds complex (Fig. 1D).
Fig. 1. Expression of FLAG-P/rds and HA-ROM-1 in Cos-7 cells.
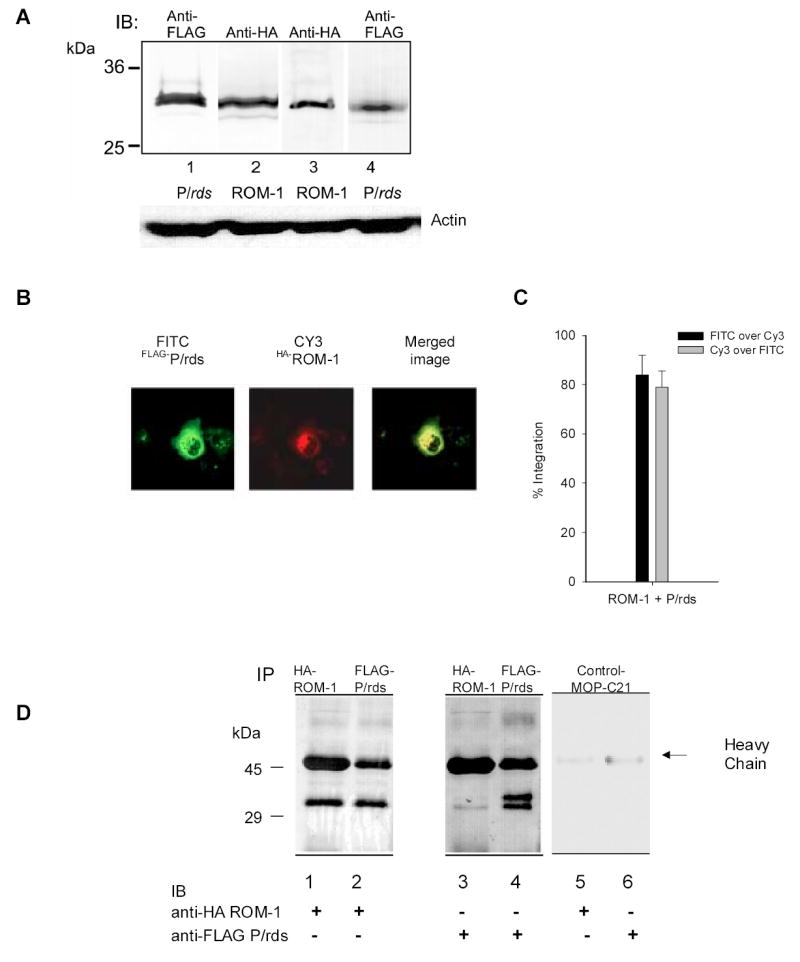
A. levels of FLAG-P/rds and HA-ROM-1 expression in COS 7 cells. Levels of P/rds and ROM-1 protein expression were compared in cell extracts prepared as described in the methods from Lane1- cells transfected with only FLAG-P/rds, Lane 2, Cells transfected with only HA-ROM-1, Lane 3- cells co-transfected with both FLAG-P/rds and HA-ROM-1, probed with anti-HA antibody and Lane 4 co-transfected cells probed with anti-FLAG Ab. Similar levels of total protein are expressed in all three transfections. Bottom portion of figure, actin protein loading controls, membranes were stripped and re-probed with 1:250 dilution of anti-actin antibody (Santa Cruz Biotechnology). B. Co-localization of FLAG-P/rds and HA-ROM-1. COS cells were co-transfected with FLAG- P/rds and HA-ROM-1, permeabilized and immuno-stained. FLAG-P/rds was detected with M5 anti-FLAG and FITC conjugated goat-anti-mouse secondary antibody. HA-ROM-1 was detected using HA polyclonal antibody CY-3 conjugated secondary Ab. All images were captured with the same laser settings. C. Quantitation of co-localization. Analysis of fluorescent probe co-localization was performed using image analysis software [Metamorph; (Universal Imaging Corporation; Downingtown, PA), ver.6]. Regions of interest were defined to include cells that did not overlap. The region was segmented to select pixels above a constant threshold value (>60% above background) which represent true fluorescence. Since both spatial location and intensity of pixels contribute to co-localization, the values represent the integrated intensity; pixels in both Cy-3 and FITC images had similar brightness values and spatial location. The average pixel intensity for each is presented as either FIOTC over Cy-3 (black bars) or Cy-3 over FITC (grey bars). Co-localization analysis was performed on all cells present in Figure 1B. D. Co-immunoprecipitation of FLAG-P/rds and HA-ROM-1. COS-7 cells transfected with FLAG-P/rds and HA-ROM-1 were harvested, cell extracts prepared and immuno-precipitated with M5 monoclonal anti-FLAG-Ab or anti-HA antibody. Immunoprecipitates were fractionated and immuno-blotted with either monoclonal anti-HA Ab (lanes 1, 2 and 5) or monoclonal anti-FLAG Ab (lanes 3, 4 and 6) as indicated. Negative MOP-C21 controls are shown in lanes 5 and 6. Marker sizes in kDa are indicated on the left.
To understand how ROM-1- P/rds containing membranes fuse with the in vivo OS target, the OS plasma membrane we analyzed the kinetics of membrane merger using fluorescence resonance energy transfer techniques (FRET). One membrane species the OS plasma membrane (PM) was labeled with R18 (R18 PM), the other the COS-7 membranes were labeled with F18. As these two fluorescently labeled membranes merge (fuse) an increase in R18 fluorescence emission is observed since the emission spectra of F18 overlaps with the excitation spectra of R18 resulting in a transfer of resonance energy from F18 to R18 as shown in the representative trace Fig. 2A. The increase in R18 fluorescence emission is linear over time and proportional to the rate of fusion between the two membranes (Boesze-Battaglia 2000).
Fig. 2. A. Resonance energy transfer between F18 and R18 labeled membranes.
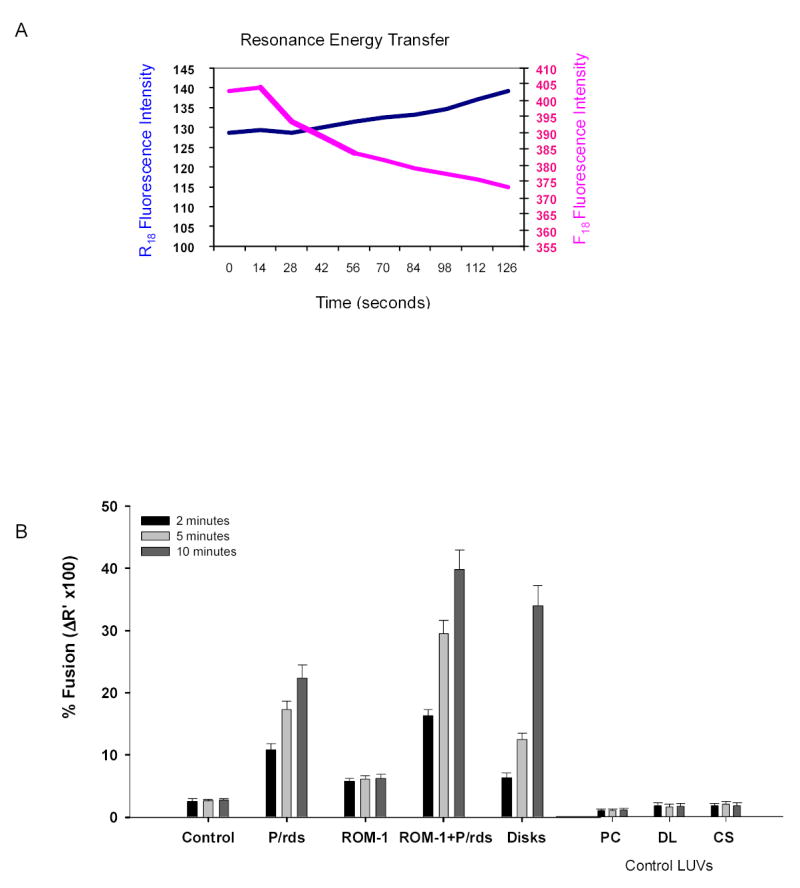
A representative fluorescence emission scan showing an increase in R18 emission (λem=592, blue line) with a concomitant decrease in F18 emission (λem=524, pink line) during the fusion of two labeled membrane species. In this particular experiment fusion between F18-labeled COS cell intracellular membranes (expressing FLAG-peripherin constructs) and R18-labeled bovine ROS plasma membrane was measured FRET as described above in the Methods All fusion assays were carried out at room temperature under dim light. B. Fusion between R18-PM and F18-target membranes. FRET- based fusion assays were used to follow fusion between R18-PM and membrane(s) isolated from Control (mock-transfected COS cells) and cells expressing P/rds, ROM-1 or P/rds + ROM-1 in co-transfection analyses. Control target membranes, phosphatiodylcholine LUV (PC LUV), disk lipid extract LUV (DL LUV) or cos cell membrane extract LUV (CS) were prepared as described in the methods. The results show the % fusion at 2 min (black bars), 5 min, (light-gray bars) and 10 minutes, (dark gray bars). All assays were compared to fusion between R18-PM and F18–disks (indicated as disks). Fusion was initiated with the addition of R18-PM to F18-target membranes in 96 well plates at RT. Data represent mean +/+ SE of three independent preparations each done in at least duplicate.
The % fusion between R18 PM and COS cell membranes was calculated at three time points, 2 min, 5 min and 10 min. After 2 minutes, minimal fusion was detected between R18 PM and COS cell membranes isolated from cells transfected with either P/rds alone or ROM-1 alone (Fig. 2B designated P/rds or ROM-1). Interestingly, even at this early 2 minute time point COS-7 cell membranes containing both P/rds and ROM-1 showed 15% fusion, a value higher than that observed with disk membranes. By ten minutes, 40% fusion was observed in membranes co-expressing P/rds and ROM-1 and in disk membranes. The extent of fusion (shown as % fusion) in membranes containing both proteins was 2-fold higher than with P/rds -containing membranes alone (Fig. 2B). No additional fusion was detected between R18PM and any of the F18 labeled target membranes after the ten minute time point. As expected membranes isolated from mock-transfected COS cells, designated controls showed virtually no fusion with R18 PM. In addition, fusion between the R18 and F18 labeled membrane species was inhibited with the addition of 10μM to 1mM EDTA (data not shown) confirming the requirement for calcium in photoreceptor fusion (Boesze-Battaglia, Albert et al. 1992) Model target membranes consisting of phosphatidylcholine large unilammelar vesicles (PC LUV), LUVs prepared from disk lipid extracts (DL LUV) or LUVs prepared from COS cell membrane extracts (CS LUV) showed no fluorescence transfer with R18 PM. Lastly, when R18 labeled PM vesicles were incubated with F18 labeled PM vesicles no fusion was detected (data not shown). These results suggest that energy transfer is accurately monitoring membrane merger and not spontaneous probe transfer and that optimal fusion likely requires a both ROM-1 and P/rds within the membrane complex.
Collectively the heterologously expressed protein studies suggest that ROM-1 may play a role in membrane fusion processes. In this second series of studies we tested the hypothesis that ROM-1 alone could promote fusion. In order to determine if ROM-1 was fusogenic, we prepared proteo-liposome target membranes consisting of large unilammelar vesicles (LUV) prepared from disk membrane lipid extracts and purified ROM-1. ROM-1 was purified from bovine ROS membranes using a combination of affinity chromatography and chromatofocusing techniques. As shown previously P/rds elutes from a chromatofocusing column at its pI (equal to 4.7), allowing ROM-1 with pI of 5.8 to be isolated independently (Boesze-Battaglia, Kong et al. 1997). This same strategy was used in the isolation of purified P/rds (Boesze-Battaglia, Kong et al. 1997). ROM-1 was isolated from a vesicle preparation enriched in P/rds using a PBE-94 column eluted with Polybuffer as described in the methods (Boesze-Battaglia, Kong et al. 1997). ROM-1 was routinely recovered in fractions #75-80, corresponding to a pH range from 5.40 to 5.32, in close agreement with its theoretical pI of 5.8. Fractions were pooled and proteins separated by SDS-PAGE. A single 30 kDa band with virtually no contaminating proteins was detected in silver stained gels (Fig. 3A, lane 2). Specificity was confirmed by western blot analysis using anti-ROM-1 mAb 1D5 (Fig 3A, lane 1).
Fig. 3. Assessment of ROM-1 fusogenicity.
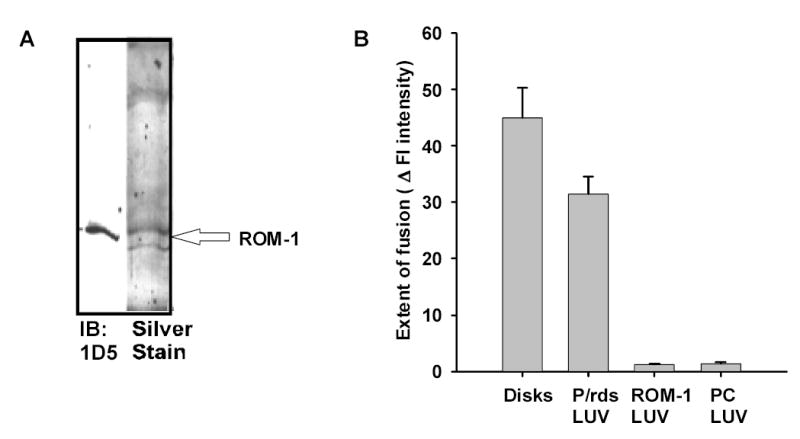
A. Isolation of ROM-1 from bovine OS membranes. ROM-1 was purified using a strategy identical to that described for P/RDS and described in detail in the Methods. Purified ROM-1 isolated in fractions 75 to 82 was run on SDS-Gels (10%) and silver stained (lane 1). An aliquot of the fraction (lane 2) was also run on SDS-PAGE and transferred to nitrocellulose and labeled with anti-ROM-1 antibody 1D5 (a generous gift from Dr. Robert Molday). B. Fusion between R18-PM and unlabeled target membranes; analysis of final extent of fusion. The extent of fusion between R18-PM and LUV containing either P/rds or ROM-1 was followed at 37oC. The extent of fusion is determined as the % change in fluorescence intensity over a 60 minute time period. During this period, fusion between R18-PM and disks goes to completion (Boesze-Battaglia, Albert et al. 1992; Boesze-Battaglia 1997). Results are the mean +/− SEM for three independent preparations each in duplicate
We compared the extent of fusion between R18 PM vesicles with disk membranes or proteo-liposomes containing either P/rds or ROM-1 prepared as described (Boesze-Battaglia 2000) or PC LUV. In contrast to the FRET based fusion assay used in our first series of studies these studies used a fluorescence dequenching assay because the relative ratio of the two membranes was not 1:1 as in the FRET but approximately 100:1 (target membrane:PM). Upon the addition of R18 PM to target membranes, R18 fluorescence is de-quenched and an increase in intensity is observed if the two membranes merge. This linear increase in intensity allows us to calculate an initial rate of fusion (IRF) and a final extent of fusion. Both disk membranes and P/rds containing LUVs showed similar final extents of fusion (Fig 3B), 45 +/− 5.3 and 31.5 +/− 3.0 respectively. ROM-1 containing LUV showed no detectable fusion, suggesting that ROM-1 alone is not fusogenic (Fig 3B).
Since ROM-1 showed no observable fusion activity we focused our efforts on understanding how ROM-1 in the presence of P/rds, as occurs in vivo affects fusion. Thus in this next series of studies we utilized ROM-1–P/rds enriched vesicles, called rim specific vesicles, (RSV) as target membranes for fusion. These vesicles are derived from solubilized bovine OS membrane fractions and are formed upon dialysis of the lipid-rich flow-through from a Concanavalin-A affinity column. We have shown that these vesicles are devoid of rhodopsin and enriched in the ROM-1-P/rds complex (Boesze-Battaglia, Kong et al. 1997). RSV have a cholesterol to phospholipid mole ratio equal to 15:1 (mole:mole) and consist of 120–180 moles of phospholipid per mole total protein. As shown in Fig 4A, these vesicles contain both P/rds and ROM-1- as detected by Western blot analysis. The C-terminus of P/rds was oriented facing the outside of the vesicle (Fig. 4B) similar to what is observed in vivo; extra-diskally.
Fig. 4. A. Western blot of ROM-1-P/rds enriched vesicles.
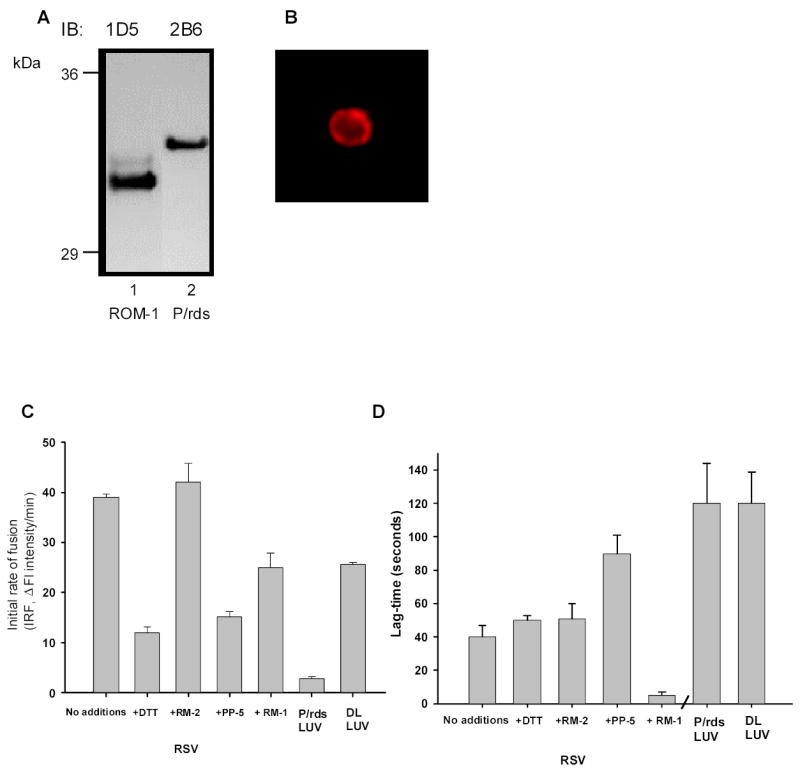
RSV were prepared as described in the Methods, separated by SDS- PAGE transferred and immunoblottted with either anit-P/RDS mAb 2B6 or anti-ROM-1 mAb 1D5. B. FITC labeling of ROM-1 P/rds Enriched Vesicles (RSV). Rim specific vesicles (RSV’s) were prepared from the dialyzed lipid rich fraction of ROS–disk Con A chromatography by freeze/thawing, labeled with anti-P/RDS mAb antibody 2B6 and imaged as described in the Methods. C. Fusion kinetics between R18-PM and unlabeled target membranes; analysis of IRF. Initial Rates of Fusion (IRF) are shown for fusion between R18-PM and disk membranes as well as RSV. RSV fusion is shown on the left. RSV –R18-PM fusion with no-additions RSV pre-incubated with 10 mM DTT, 10μg/ml PP-5 peptide or 10 μg/ml RM-1 or RM-2 peptide as indicated. Results are the mean +/− SEM for three independent preparations each in duplicate. Controls showing fusion between R18-PM and either P/rds containing LUV or DL LUV is indicated on the right in the last two columns. D. Fusion kinetics between R18-PM and unlabeled target membranes; analysis of lag-time. Lag-times are shown for fusion between R18-PM and RSV on the left. Lag-times observed when RSV fuse with R18-PM fusion with no-additions or RSV pre-incubated with 10 mM DTT, 10μg/ml PP-5 peptide or 10 μg/ml RM-1 or RM-2 peptide as indicated. Results are the mean +/− SEM for three independent preparations each in duplicate. Controls showing lag-time when fusion between R18-PM and either P/rds containing LUV or DL LUV is indicated on the right in the last two columns. Results are the mean +/− SEM for three independent preparations each in duplicate.
Again we compared the fusion kinetics between R18 PM and various target membranes (Fig. 4C) using the R18 dequenching assay. As mentioned, prior to the linear increase in R18 intensity a lag-time is observed. This time is indicative of the time it takes to assemble a fusion complex. Both initial rates of fusion and lag-times were determined in mixtures of R18 PM and target membranes. Most interesting is the observation that RSV containing both P/rds and ROM-1 in a native membrane lipid environment, (i.e., disk lipids), showed the highest initial rates of fusion (Fig 4C). Fusion between R18 PM and all of the target membranes was inhibited with the addition of the fusion inhibitory peptide, PP-5 as described previously (Fig. 4C) and anti- P/rds mAb 2B6 (data not shown).
To understand how ROM-1 and P/rds may cooperate to form a fusion competent complex we focused our analyses on the RSV. In this next series of studies we analyzed two kinetic components of this fusion, the initial rate of fusion and lag-time prior to the initiation of fusion under conditions that either disrupt subunit assembly (DTT or RM-2 peptide), target the C-terminal domain of ROM-1 (RM-1 peptide) or target the C-temrnal domain of P/rds. When RSV were pre-incubated with the reducing agent, DTT (at 1mM), the IRF decreased by 50% (Fig 4D). However, little change in IRF was detected when the RSV were preincubayed with RM-2, a peptide mimicking the region of P/rds that is invoel din P/rds- ROM_1 hetre-oligmeirazaiotn. In addition, when RSV were pre-incubated with RM-1 299VIDGEGEAQGYLFPG314 a peptide corresponding to the most highly conserved region between P/rds and the ROM-1 C-termini, no change in the IRF was observed (Fig 4D). Collectively, these results and those shown in Fig 4C suggest that ROM-1 is most likely not directly involved in the fusion process.
A comparison of the lag-times observed in these fusion assay mixtures suggests that ROM-1 may mediate a fusion competent form of P/rds. As shown in Figure 4D, the lag-time observed with RSV is 3 fold lower than that observed with P/rds LUVs. This is a significant decrease since the ratio of phospholipid to protein in both complexes is similar, 120–180 moles phospholipid to protein in RSV and 120:1 in P/rds LUV. Thus the ability to laterally diffuse or create a membrane rich fusion surface appears not to be a trivial reason for the substantial decrease in lag-time. The addition of the P/rds inhibitory peptide, PP-5 decreased lag-time while the addition of RM-1 almost completely abolished the lag-time. Thus it appears that fusion between R18 PM and RSV occurs optimally in the presence of ROM-1 and fusion between these membranes likely requires the C-terminus of ROM-1 possibly as a component of a fusion complex.
Discussion
Photoreceptor renewal requires a number of distinct fusion events including the fusion of OS plasma membrane with disk membranes during disk shedding. Fusion between these two membranes requires calcium (Boesze-Battaglia, Albert et al. 1992), is stimulated by physiological concentrations of retinal/ol (Boesze-Battaglia, Fliesler et al. 1992) and is likely mediated by an P/RDS containing fusion complex (Boesze-Battaglia, Lamba et al. 1998). Initial studies using a ROM-1- P/rds co-transfection system clearly showed that in the presence of ROM-1 membrane fusion with OS plasma membrane was greatly enhanced. To understand how ROM-1 modifies P/rds dependent fusion we analyzed various aspects of the kinetics of fusion between R18PM and various target membranes. One kinetic component, the lag-time has provided valuable insight into the mechanism by which ROM-1 may contribute to fusion. Lag-times are components of most fusion systems and reflect the time it takes for a fusion complex to become fully-fusion competent. Often fusion competency requires a rapid conformational change of the fusion protein as well as the formation of a fusion complex. Such complex assembly often relies on the lateral diffusion of components of the fusion machinery to the site of fusion subsequent to receptor binding. The effect of ROM-1 on the lag-time was confirmed in in vitro studies using various proteo-liposomes, including purified ROM-1 containing LUVS and ROM- P/rds enriched RSV. In the cell free assays, the lag-times observed in the presence of ROM-1 were much shorter than with P/rds alone, although ROM-1 itself did not behave as a membrane fusion protein in a manner analogous to P/rds. Interestingly when ROM-1 and P/rds are in their most “native-like” environment in RSV, fusion is the most robust, suggesting that ROM-1 although not essential aids in promoting fusion.
Although it has been clearly shown that the EC2 of P/rds is the site of subunit assembly (Goldberg, Fales et al. 2001) and the determinants involved in this assembly have be mapped to residues Cys165-Asn182 within the N-terminal portion of this domain (Phe120-Phe187) (Ding, Stricker et al. 2005), the importance of subunit assembly for optimal fusogenic function is not clear. Goldberg, et al., have shown that targeting and subunit assembly proceed normally in the absence of fusion activity (Goldberg, Fales et al. 2001). As a corollary, we observed that mutants showing altered subunit assembly, defined here as the formation of P/rds tetramers, were unable to promote model membrane fusion (Boesze-Battaglia and Stefano 2002). In the present studies we show that pre-treatment of target membranes (RSV) with DTT resulted in a decrease in IRF and increased lag-time. Peptide competition studies in which a peptide corresponding to the EC-2 binding domain Cys 165-Asn182, called RM-2 had no effect on fusion. This is not entirely unexpected since the peptide is not membrane permeant and may not alter ROM-1- P/rds interactions under the conditions necessary to measure in vitro fusion. Collectively, these results suggest that P/rds monomers and/or homodimers or ROM-1- P/rds hetero-dimers are only minimally fusogenic.
The mechanism by which ROM-1 potentiates fusion is speculative. Peptide competition studies suggest that a portion of the ROM-1 C-terminus (RM-1, residues 299 to 311) aids in promoting a fusion–competent P/rds, likely through a ROM-1- P/rds interaction. An interaction between P/rds and ROM-1 was proposed previously by Travis, et al., (Travis, Sutcliffe et al. 1991) although GST pull-down experiments failed to confirm this observation (Ding, Stricker et al. 2005). Lastly, ROM-1 may serve as a fusion cofactor or as a co-receptor in a manner analogous to glycoprotein D (gD) in HSV fusion (Terry-Allison, RI et al. 1998) or the chemokine receptors, CCR5 and CXCR4 in HIV-mediated fusion (Doranz, Orsini et al. 1999). Previous work in the lab has suggested that ROM-1 interacts with a 60–65 kDa plasma membrane specific ricin binding protein, currently the identity of this protein is under investigation. Independent of the precise mechanism by which ROM-1 participates in fusion, these studies clearly suggest that it is part of a photoreceptor specific fusion complex. The formation of a fusion complex is consistent with the recent identification of a potent endogenous inhibitor of OS fusion, melanoeregulin, as well as these studies and others showing that while inhibitory neither the RM-1 peptide or PP-5 can completely abolish fusion.
The phenotype of the ROM-1 knockout mouse, large-disorganized OS, led Clarke, et al., 2000 to propose that ROM-1 is required for the regulation of disk morphogenesis (Clarke, Goldberg et al. 2000). P/rds is detected within the photoreceptor OS of the ROM-1 knockout mouse and localized to the periphery analogous to the localization pattern observed in wild type (Clarke, Goldberg et al. 2000). It is likely that ROM-1 plays an accessory role in disk morphogenesis and may regulate the formation of a fusion complex as indicted in these studies. This hypothesis is further reinforced by the localization pattern of ROM-1 in rds −/− and rds +/− mice. In this mouse models of human RP, ROM-1 is concentrated primarily in the putative outer segment domains, along the distal connecting cilium suggesting that it incorporates into the OS membrane in the absence of P/rds (Lee, Burnside et al. 2006). Collectively these animal models suggest a dominant role for P/rds in morphogenesis.
The paradigm or more accurately, paradigm(s) one evokes to model photoreceptor specific fusion events is somewhat controversial. There are in fact a whole series of tightly regulated membranes fusion events that must occur during renewal. These include fusion associated with the proper delivery of materials from the IS, fusion during closure of disks upon morphogenesis and lastly, fusion required for shedding and phagocytosis of spent OS. Whether one considers disk closure as a cell-cell fusion event, a process for which little is known of the mechanistic underpinnings, or a paradigm of v-SNARE, t-SNARE fusion, clearly the design and development of good cell free assay system(s) to complement in vivo and microscopic analysis is essential.
Acknowledgments
This work was supported by U.S.P.H.S. grant EY10420, an E. Matilda Ziegler Vision Award and a Vision Core Grant P30 EY001583.
References
- Bartlett GR. Phosphorous assay in column chromatography. J Biol Chem. 1959;234:466–473. [PubMed] [Google Scholar]
- Boesze-Battaglia K. Fusion of intracellular rod outer segment disk membranes with the surrounding plasma membrane. Invest Ophthalmol Vis Sci. 1997;38:18–26. [PubMed] [Google Scholar]
- Boesze-Battaglia K. Fusion between retinal rod outer segment membranes and model membranes: Functional assays and a role for peripherin/rds. Methods Enzymol. 2000;316:65–87. doi: 10.1016/s0076-6879(00)16717-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Albert AD, et al. Fusion between disk membranes and plasma membranes of bovine photoreceptor cells is calcium dependent. Biochemistry. 1992;31:3733–3737. doi: 10.1021/bi00130a002. [DOI] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Fliesler SJ, et al. Retinal and retinol promote membrane fusion. Biochim Biophys Acta. 1992;1111:256–262. doi: 10.1016/0005-2736(92)90318-g. [DOI] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Goldberg AF. Photoreceptor renewal: a role for peripherin/rds. International Review of Cytology. 2002;217:183–225. doi: 10.1016/s0074-7696(02)17015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Kong F, et al. Purification and light dependant phosphorylation of a candidate fusion protein, photoreceptor peripherin/rds. Biochemistry. 1997;22:6835–6846. doi: 10.1021/bi9627370. [DOI] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Lamba OP, et al. Fusion between retinal rod outer segment membranes and model membranes; a role for photoreceptor peripherin/rds. Biochemistry. 1998;37:9477–9487. doi: 10.1021/bi980173p. [DOI] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Stefano FP. Peripherin/rds fusogenic function correlates with subunit assembly. Experimental Eye Research. 2002;75:227–31. doi: 10.1006/exer.2002.2004. [DOI] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Stefano FP, et al. A peptide analogue to a fusion domain within photoreceptor peripherin/rds promotes membrane adhesion and destabilization. Biochim Biophys Acta. 2000;1463:343–354. doi: 10.1016/s0005-2736(99)00226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Yeagle PL. Rod outer segment disc membranes are capable of fusion. Invest Ophthalmol Vis Sci. 1992;33:484–493. [PubMed] [Google Scholar]
- Chen C, Yunhai J, et al. Dynamic Behavior of Rod Photoreceptor Disks. Biophys J. 2002;83:1403–1412. doi: 10.1016/S0006-3495(02)73911-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T, Peachey NS, et al. The effect of peripherin/rds haploinsufficiency on rod and cone photoreceptors. Journal of Neuroscience. 1997;17:8118–28. doi: 10.1523/JNEUROSCI.17-21-08118.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang J, Vega C, et al. Structrual and functional impairment of endocytic pathways by retinitis pigmentosa mutant rhodopsin-arrestin complexes. J Clin Invest. 2004;114:131–40. doi: 10.1172/JCI21136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G, Goldberg AF, et al. Rom-1 is required for rod photoreceptor viability and the regulation of disk morphogenesis. Nat Genet. 2000;25:67–73. doi: 10.1038/75621. [DOI] [PubMed] [Google Scholar]
- Connell GJ, Bascom R, et al. Photoreceptor peripherin is the normal product of the gene responsible for retinal degeneration in the rds mouse. Proc Natl Acad Sci USA. 1991;88:723–726. doi: 10.1073/pnas.88.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell GJ, Molday RS. Molecular cloning, primary structure, and orientation of the vertebrate photoreceptor cell protein peripherin in the rod outer segment disk membrane. Biochemistry. 1990;29:4691–4698. doi: 10.1021/bi00471a025. [DOI] [PubMed] [Google Scholar]
- Deretic D, Papermaster D. Rab6 is associated with a compartment that transports rhodopsin from the trans-Golgi to the site of rod outer segment disk formation in frog retinal photoreceptors. J Cell Sci. 1993;106:803–813. doi: 10.1242/jcs.106.3.803. [DOI] [PubMed] [Google Scholar]
- Deretic D, Papermaster D. Progress in Retinal and Eye Research. New York; Pergamon Press: 1995. [Google Scholar]
- Deretic D, Papermaster DS. Polarized sorting of opsin on post-Golgi membranes in retinal photoreceptor cells. J Cell Biol. 1991;113:1281–1293. doi: 10.1083/jcb.113.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding XQ, Stricker HM, et al. Role of the second intradiscal loop of peripherin/rds in homo and hetero associations. Biochemistry. 2005;44:4897–904. doi: 10.1021/bi048414i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doranz BJ, Orsini M, et al. Identification of CXCR4 Domains That Support Coreceptor and Chemokine Receptor Functions. J Virol. 1999;73(4):2752–2761. doi: 10.1128/jvi.73.4.2752-2761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AF, Molday RS. Subunit composition of the peripherin/rds-rom-1 disk rim complex from rod photoreceptors: Hydrodynamic evidence for a tetrameric quaternary structure. Proc Natl Acad of Sci. 1996;35:6144–6149. doi: 10.1021/bi960259n. [DOI] [PubMed] [Google Scholar]
- Goldberg AF, Moritz OL, et al. Heterologous expression of photoreceptor peripherin/rds and Rom-1 in COS-1 cells: Assembly, interactions, and localization of multisubunit complexes. Biochemistry. 1995;34:14213–14219. doi: 10.1021/bi00043a028. [DOI] [PubMed] [Google Scholar]
- Goldberg AF, Ritter LM, et al. Structure and activity of non-fusogenic peripherin/rds in transgenic rod photoreceptors 2006 [Google Scholar]
- Goldberg AFX, Fales LM, et al. Folding and subunit assembly of photoreceptor peripherin/rds is mediated by determinants within the extracellular/intradiskal EC2 domain. J Biol Chem. 2001;276:42700–42706. doi: 10.1074/jbc.M107511200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AFX, Molday RS. Defective subunit assembly underlies a digenic form of retinitis pigmentosa linked to mutations in peripherin/rds and rom-1. Proc Natl Acad Sci USA. 1996;93:13726–13730. doi: 10.1073/pnas.93.24.13726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RK, Jansen HG, et al. Development and degeneration of retina in rds mutant mice: Photoreceptor abnormalities in the heterozygotes. Exp Eye Res. 1985;41:701–720. doi: 10.1016/0014-4835(85)90179-4. [DOI] [PubMed] [Google Scholar]
- Hoekstra D, Boer TD, et al. Fluorescence method for measuring the kinetics of fusion between biological membranes. Biochemistry. 1984;23:5675–5681. doi: 10.1021/bi00319a002. [DOI] [PubMed] [Google Scholar]
- Hoekstra D, Klappe K. Sendai virus-erythrocyte membrane interaction: Quantitative and kinetic analysis of viral binding, dissociation and fusion. J Virol. 1986;58:87–95. doi: 10.1128/jvi.58.1.87-95.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara K, Berson EL, et al. Digenic retinitis pigmentosa due to mutations at the unlinked peripherin/RDS and ROM 1 loci. Science. 1994;264:1604–1608. doi: 10.1126/science.8202715. [DOI] [PubMed] [Google Scholar]
- Kedzierski W, Weng J, et al. Analysis of the rds/peripherin.rom1 complex in transgenic photoreceptors that express a chimeric protein. J Biol Chem. 1999;274:29181–29187. doi: 10.1074/jbc.274.41.29181. [DOI] [PubMed] [Google Scholar]
- Körschen HG, Beyermann M, et al. Interaction of glutamic-acid-rich proteins with the cGMP signalling pathway in rod photoreceptors. Nature. 1999;400:761–766. doi: 10.1038/23468. [DOI] [PubMed] [Google Scholar]
- Lee ES, Burnside B, et al. Characterization of Peripherin/rds and ROM-1 Transport in Rod Photoreceptors of Transgenic and Knockout Animals. Invest Ophthalmol Vis Sci. 2006;47:2150–2160. doi: 10.1167/iovs.05-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman BJ. Lipid model membranes. Characterization of mixed phospholipid vesicles. Biochemistry. 1973;12:2545–2554. doi: 10.1021/bi00737a028. [DOI] [PubMed] [Google Scholar]
- Litman BJ. Methods Enzymol. 1982;81:150. doi: 10.1016/s0076-6879(82)81025-2. [DOI] [PubMed] [Google Scholar]
- Loewen CJ, Moritz O, et al. Molecular characterization of peripherin-2 and rom-1 mutants responsible for digenic retinitis pigmentosa. J Biol Chem. 2001;276:22388–22396. doi: 10.1074/jbc.M011710200. [DOI] [PubMed] [Google Scholar]
- McNally N, Kenna PF, et al. Murine model of autosomal dominant retinitis pigmentosa generated by targeted deletion at codon 307 of the rds-peripherin gene. Human Molecular Genetics. 2002;11:1005–16. doi: 10.1093/hmg/11.9.1005. [DOI] [PubMed] [Google Scholar]
- Moritz OL, Molday RS. Molecular cloning, membrane topology, and localization of bovine rom-1 in rod and cone photoreceptor cells. Invest Ophthalmol Vis Sci. 1996;37:352–362. [PubMed] [Google Scholar]
- Muller-Weeks S, Boesze-Battaglia K, et al. Deletional analysis of the rod photoreceptor cell peripherin/RDS carboxy-terminal region. Experimental Eye Research. 2002;75:143–54. doi: 10.1006/exer.2002.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oprian D. Expression of opsin genes in COS cells. In: Hargrave P, editor. Methods in Neuroscience. San Diego: Academic Press; 1993. pp. 301–306. [Google Scholar]
- Partearroyo MA, Cabezon E, et al. Real-time measurements of chemically induced membrane fusion in cell monolayers, using a resonance energy transfer method. Biochim Biophys Acta. 1994;1189:175–180. doi: 10.1016/0005-2736(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Poetsch A, Molday LL, et al. The cGMP-gated channel and related glutamic acid rich proteins interact with peripherin-2 at the rim region of rod photoreceptor disc membranes. J Biol Chem. 2001;276:48009–48016. doi: 10.1074/jbc.M108941200. [DOI] [PubMed] [Google Scholar]
- Sanyal S, Hawkins RK. Development and degeneration of retina in rds mutant mice: Altered disk shedding pattern in the albino heterozygotes and its relation to light exposure. Vision Res. 1988;28:1171–1178. doi: 10.1016/0042-6989(88)90034-x. [DOI] [PubMed] [Google Scholar]
- Sanyal S, Jansen HG. Absence of receptor outer segments in the retina of rds mutant mice. Neurosci Lett. 1981;21:23–26. doi: 10.1016/0304-3940(81)90051-3. [DOI] [PubMed] [Google Scholar]
- Smith HG, Stubbs GW, et al. Exp Eye Res. 1975;20:211–219. doi: 10.1016/0014-4835(75)90134-7. [DOI] [PubMed] [Google Scholar]
- Steinberg RH, Fisher SK, et al. Disc morphogenesis in vertebrate photoreceptors. J Comp Neurol. 1980;190:501–508. doi: 10.1002/cne.901900307. [DOI] [PubMed] [Google Scholar]
- Tam BM, Moritz OL, et al. Are the COOH terminal regions for rod outer segment proteins potential targeting signals? Invest Ophthalmol Vis Sci. 2001;42(4) [Google Scholar]
- Tam BM, Moritz OL, et al. Characterization of the Functional Properties of the C-terminus of Xenopus Peripherin. Investigative Ophthalmology & Visual Science. 2002;43(4) [Google Scholar]
- Tam BM, Moritz OL, et al. The C terminus of peripherin/rds participates in rod outer segment targeting and alignment of disk incisures. Mol Biol Cell. 2004;15(4):2027–37. doi: 10.1091/mbc.E03-09-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry-Allison T, RI M, et al. HveA (Herpesvirus Entry Mediator A), a Coreceptor for Herpes Simplex Virus Entry, also Participates in Virus-Induced Cell Fusion. J Virol. 1998;72(7):5802–5810. doi: 10.1128/jvi.72.7.5802-5810.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, et al. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Nat Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis GH, Brennan OL, et al. Identification of a photoreceptor-specific mRNA encoded by the gene responsible for retinal degeneration slow (rds) Nature. 1989;338:70–73. doi: 10.1038/338070a0. [DOI] [PubMed] [Google Scholar]
- Travis GH, Sutcliffe JG, et al. The retinal degeneration slow (rds) gene product is a photoreceptor disc membrane-associated glycoprotein. Neuron. 1991;6:61–70. doi: 10.1016/0896-6273(91)90122-g. [DOI] [PubMed] [Google Scholar]


