Abstract
Type 1 diabetes is characterized by T cell-mediated autoimmune destruction of pancreatic β cells. Several studies have suggested an association between Coxsackie enterovirus seroconversion and onset of disease. However, a direct link between β cell viral infection and islet inflammation has not been established. We analyzed pancreatic tissue from six type 1 diabetic and 26 control organ donors. Immunohistochemical, electron microscopy, whole-genome ex vivo nucleotide sequencing, cell culture, and immunological studies demonstrated Coxsackie B4 enterovirus in specimens from three of the six diabetic patients. Infection was specific of β cells, which showed nondestructive islet inflammation mediated mainly by natural killer cells. Islets from enterovirus-positive samples displayed reduced insulin secretion in response to glucose and other secretagogues. In addition, virus extracted from positive islets was able to infect β cells from human islets of nondiabetic donors, causing viral inclusions and signs of pyknosis. None of the control organ donors showed signs of viral infection. These studies provide direct evidence that enterovirus can infect β cells in patients with type 1 diabetes and that infection is associated with inflammation and functional impairment.
Keywords: Coxsackie B4 enterovirus, type 1 diabetes
Type 1 diabetes mellitus is believed to result from the selective autoimmune destruction of pancreatic islet β cells, occurring in genetically predisposed subjects, and possibly triggered or accelerated by environmental agents (1–3). One of the environmental risk factors identified by several independent studies is represented by enteroviral infection (4). Epidemiological data showed an increased incidence of type 1 diabetes after epidemics due to enteroviruses, and enteroviral RNA has been detected in the blood of >50% of type 1 diabetes patients at the time of disease onset (5).
Coxsackie virus B4 has been isolated from patients with acute onset type 1 diabetes (6), and some of these isolates have been reported to cause diabetes in mice (7). In cultured human islet cells, several enterovirus strains can replicate, suppress insulin release, and, in a few cases, cause β cell destruction (8).
Enteroviruses may trigger or accelerate the pathological events leading to clinical type 1 diabetes by several mechanisms, which are not necessarily mutually exclusive (4, 7, 8). First, infected islet β cells could be directly destroyed by virus-induced cytolysis. Second, a less aggressive infection could cause an inflammatory reaction in the islets, leading to subclinical levels of β cell destruction and subsequent release of normally sequestered antigens, which then trigger pathogenic autoreactive T cell responses. Alternatively, cross-reactive T cells could be induced, which occurs when viral antigens and self-antigens share antigenic determinants (9, 10).
To date, a direct link between β cell enterovirus infection in vivo, β cell dysfunction, islet inflammation, and β cell destruction has not been established in humans, although signs of enteroviral infection have been recently reported in a small subset of pancreata obtained at autopsy from type 1 diabetic subjects (11). In search for the relationship between viral infection, insulitis, autoimmunity, and β cell function and survival in type 1 diabetes, we studied islets from six recent-onset type 1 diabetic patients (Table 1) collected over a period of 4 years and from 26 control multiorgan donors. Indeed we found that β cells from three of six diabetic patients showed signs of enteroviral infection associated with natural killer (NK) cell islet infiltration. Virus was isolated from infected islets of a patient, sequenced, and identified as a Coxsackie B4. In addition, isolated virus was able to in vitro infect β cells from nondiabetic multiorgan donors, causing β cell dysfunction characterized by impaired glucose-stimulated insulin release.
Table 1.
Characteristics of type 1 diabetic patients studied
| Patient no. | Age, yr | Sex | Time from diagnosis | β cell function | Enteroviral infection of β cells | β cell destruction | Insulitis |
|---|---|---|---|---|---|---|---|
| 1 | 26 | F | —* | Impaired | Yes | No | Minor; dominated by NK cells; no evidence for autoreactive T cells |
| 2 | 19 | M | 9 months | Partially lost | Yes | No | Minor; dominated by NK cells; no evidence for autoreactive T cells |
| 3 | 15 | F | 1 week | Lost | Yes | Limited | Minor; dominated by NK cells; no evidence for autoreactive T cells |
| 4 | 14 | F | 8 months | Lost | No | Yes | Moderate T cell infiltrate; no NK cells |
| 5 | 5 | M | 1 week | Lost | No | Yes | Moderate T cell infiltrate; no NK cells |
| 6 | 4 | F | 1 week | Lost | No | Yes | Moderate T cell infiltrate; no NK cells |
M, male; F, female.
*Patient 1 was a recipient of a whole pancreas graft, which was removed after 38 months because of recurrent urinary tract infections.
Results
Nondestructive Insulitis with NK Cell Infiltration.
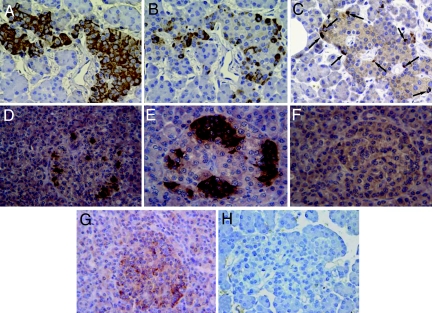
Islets of patients 1–3 were well preserved with a modest-to-moderate infiltrate of mononuclear cells affecting 74 of 112 (66%) of islets analyzed. In these patients no apparent reduction of islet β cells was seen, with insulin-positive cells representing the majority of endocrine cells in 125 of 126 islets studied (Fig. 1A). This was confirmed by electron microscopy showing that the number of β cells in islets from patients 1 and 2 (68 ± 5% of islet endocrine cells) was similar to that from three age-matched, nondiabetic controls (64 ± 5%). Conversely, in patients 4–6 a marked reduction of islet β cells was evident (Fig. 1 D–F).
Fig. 1.
A type 1 diabetic pancreas shows nondestructive insulitis with NK cell infiltration and IFNα expression. (A–C) Immunohistochemistry panel showing reactivity for insulin (A), NK cells (B), and CD3+ cells (C) in consecutive sections representing the same islet; arrows point to scattered CD3+ cells (patient 1). (D–F) Reactivity to insulin in pancreatic sections from three additional type 1 diabetic patients (patients 4–6) showing a significant reduction of insulin-positive cells in these three individuals. Positivity for IFNα was detected in patient 1 (G) and in patients 2 and 3 (not shown), but not in control individuals (H). (Magnification: ×250.)
In patients 1–3 the mononuclear cell infiltrate was composed mainly of CD94-positive (NK) cells and, to a lesser extent, of T lymphocytes (Fig. 1 B and C), with occasional B lymphocytes and CD68+ cells. In contrast, in cases 4–6 NK cells were not observed among the moderate infiltrates of CD45RO+ cells. No double-positive cells for CD94 and CD45RO were detected in any of the pancreases analyzed (data not shown), thus confirming that CD94-positive cells, where observed, were indeed NK cells and did not belong to that small subset of T lymphocytes that may express CD94. IFNα-positive cells were detected in pancreatic islets from patients 1–3 but not from patients 4–6 or from any control pancreata (Fig. 1 G and H), suggesting ongoing or previous islet viral infection.
β Cells Are Specifically Infected by Enterovirus.
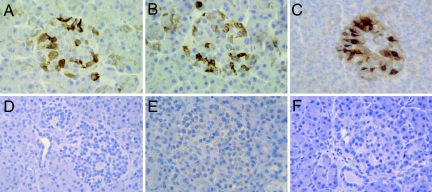
In light of serological data from patients 1 and 2, indicating positivity for Coxsackie B2 and for Coxsackie B4, we looked for islet VP-1 expression, a capside protein expressed by members of the enterovirus family. Strong VP-1 staining was observed in the majority of pancreatic islets of patients 1–3 (Fig. 2 A–C) and in few scattered exocrine cells. This VP-1-positive immunostaining was associated with a NK-dominated mild insulitis (Table 1). No VP-1 was detected in pancreatic sections from the other three type 1 diabetic patients or from 26 control organ donors (Fig. 2 D–F). VP-1 colocalized with insulin (Fig. 3 A–C) but not glucagon (Fig. 3 D–F), indicating a β cell-specific enterovirus tropism in the pancreatic islets observed. Most but not all insulin-positive cells stained positive for VP-1.
Fig. 2.
Reactivity to VP-1 enteroviral peptide in type 1 diabetic islet cells. (A–C) Immunohistochemical analysis shows reactivity to VP-1 enteroviral peptide in pancreatic islet cells from three different type 1 diabetic organ donors (patients 1–3). No VP-1 reactivity was observed in islets from another new-onset type 1 diabetic patient (D, patient 5) or in islets from control organ donors (E and F). (Magnification: ×250.)
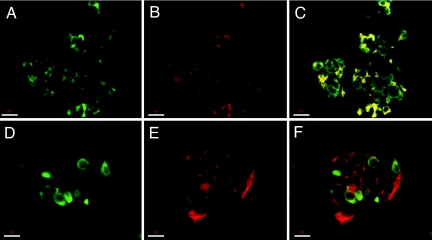
Fig. 3.
Enteroviral infection affects only β cells in pancreatic islets. Confocal microscopy analysis demonstrated that β cells (A–C), but not α cells (D–F), result positive for VP-1 staining. Cells stained in green are positive for insulin (A and C) and glucagon (D and F), those stained in red are positive for VP-1 (B, E, and F), and those stained in yellow are double-positive cells for insulin and VP-1 (C). Double-positive cells for glucagon and VP-1 were not detected (F). (Scale bars: 18μm.)
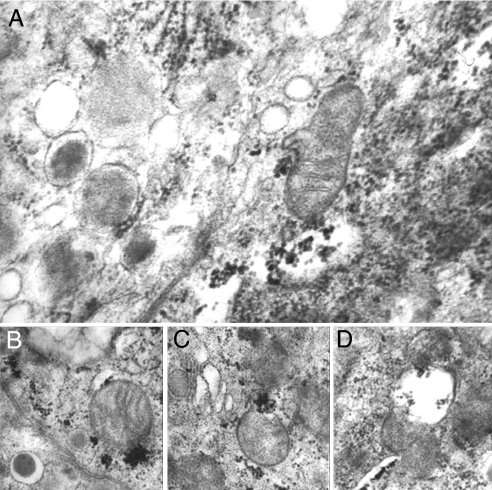
At electron microscopy (Fig. 4), viral inclusions were specifically located in the cytoplasm of pancreatic β cells of VP-1-positive islets, without alterations in α or δ cells. The percentage of infected β cells, determined by electron microscopy, ranged from 76% to 88%. Various degrees of cytopathic effects were observed, from almost intact cells to organelle disruption and cellular membrane damage, although no morphological sign of apoptosis was seen. Virus particles were abundant in areas close to mitochondria (Fig. 4A), many of which appeared swollen (Fig. 4B) or severely damaged (Fig. 4 C and D). Approximately 40% of β cells showed distorted and wrinkled nuclei, suggestive of pyknosis. Conversely, no viral inclusions were detected in pancreatic sections from any of the control organ donors.
Fig. 4.
Type 1 diabetic pancreas shows signs of viral infection. Electron microscopy of human islet β cells from a type 1 diabetic pancreas (patient 2): Many virus particles are present in the cytoplasm (A), frequently close to mitochondria (B and C) that sometimes appear swollen and partially destroyed (D). (Magnification: ×70,000.)
Enterovirus-Infected Islets Show in Vitro Loss of β Cell Function.
Islets isolated from two infected pancreata (patients 1 and 2) and from three age-matched healthy control glands were analyzed for function. Insulin content was similar in diabetic and control islets (116 ± 16 vs. 127 ± 15 microunits per islet). However, insulin release in response to glucose, arginine, and glibenclamide was lower from islets obtained from type 1 diabetic glands compared with control islets. In fact, insulin secretion (expressed as percentage of insulin content) in control and infected islets was, respectively, 1.5 ± 0.3 and 1.5 ± 0.4 at 3.3 mM glucose, 4.1 ± 1.0 and 1.6 ± 0.4 at 16.7 mM glucose (P = 0.002), 3.3 ± 0.5 and 1.8 ± 0.3 at 3.3 mM glucose plus 20 mM arginine (P = 0.003), and 3.8 ± 1.0 and 2.1 ± 0.4 at 3.3 mM glucose plus 100 μM glibenclamide (P = 0.003).
Isolated Virus Is a Coxsackie B4.
Virus was extracted from islets of case 2 and subjected to whole-genome sequencing. An unambiguous viral genome sequence of 7,395 nt was assembled and deposited in the GenBank database (accession no. DQ480420). Coxsackie B4 genome recovered (Tuscany isolate) codes for a 2183-aa polyprotein. Through BlastN and BlastP against Coxsackie B4 JVB strain (X05690), 7,366 of 7,395 (99%) and 2,171 of 2,183 (99%) identities were observed, respectively. Amino acid changes interested the VP2 region (K207E, D213G, and E214D), the P3A region (S1458R, E1459Q, V1461I, T1478I, and I1485V), the protease P3C region (Q1656R and A1711G), and the polymerase P3D region (S1781F and V1925I). That polyprotein was aligned by ClustalW [supporting information (SI) Fig. 6A] with homologues from Coxsackie virus strains previously shown to be able to in vitro infect human islets (Coxsackie A9-Griggs, B3-Nancy, B4-E2, B4-JVB, and B5-Faulkner). Sequences were analyzed to build a maximum-likelihood phylogeny. The tree topology and branch lengths are highly conserved (SI Fig. 6B).
Isolated Virus Infects Human Pancreatic β Cells in Vitro and Impairs Glucose-Stimulated Insulin Secretion.
Virus isolated from islets of patient 2 was expanded in KB cells and used to infect islets. Virus infection and replication were demonstrated in human islets of 10 nondiabetic multiorgan donors. By electron microscopy, viral inclusions were observed in the cytoplasm of 17 ± 7% and 33 ± 14% of β cells after 4 and 7 days of coculture, respectively; this finding was confirmed by VP-1 staining (SI Fig. 7).
In addition, insulin secretion in response to glucose (3.3 mM and 16.7 mM) was assessed by static incubation method and perifusion procedure. Insulin secretion (expressed as percentage of insulin content) in control and infected islets was, respectively, 1.7 ± 0.5 and 1.5 ± 0.6 at 3.3 mM glucose (not significant) and 3.8 ± 0.7 and 2.8 ± 0.5 at 16.7 mM glucose (P = 0.02).
Infected and Infiltrated Islets Express and Secrete IL-10 and TNF-α.
Islets isolated from two infected samples (patients 1 and 2) and from three age-matched healthy control glands were analyzed for cytokines. IL-4, IL-10, IFN-γ, TGF-β, and TNF-α mRNA expression was analyzed by real-time quantitative PCR, whereas cytokine secretion was determined by ELISA in cultured islets. IL-10 and TNF-α were the only cytokines detected in diabetic islets by both RT-PCR and ELISA. Real-time quantitative PCR showed mRNA for IL-10 (0.11 ± 0.01% of β-actin) and TNF-α (0.47 ± 0.03% of β-actin). IL-10 (27.9 pg/ml) and TNF-α (194.6 pg/ml), but not IFN-γ, IL-4, or TGF-β, were detected by ELISA in supernatants of cultured diabetic islets. None of these cytokines were detectable in islets from the three nondiabetic control organ donors by RT-PCR, real-time quantitative PCR, or ELISA.
No T Cell Autoreactivity in Islet-Infiltrating Lymphocytes from the Explanted Pancreas.
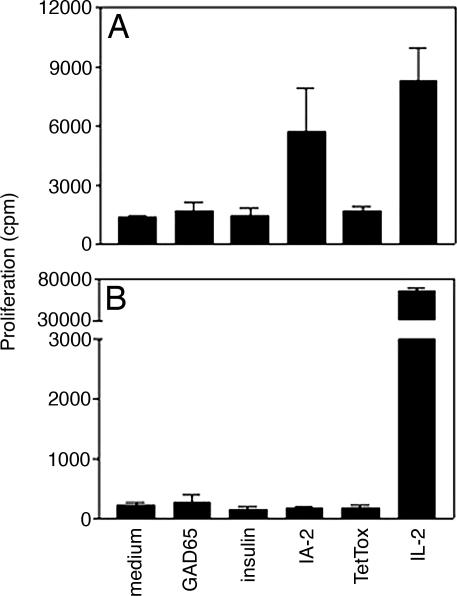
We characterized the cellular autoimmune response both in peripheral blood and in islet-infiltrating lymphocytes of case 1. Autoreactivity in peripheral blood mononuclear cells (PBMC) was restricted to the autoantigen IA-2 (Fig. 5A), which mirrored the exclusive presence of autoantibodies against this β cell determinant. A T cell line was generated that was restricted by the disease predisposing HLA-DRB1*0401. The epitopes recognized included peptides previously identified as immunodominant epitopes and naturally processed peptides of IA-2 (spanning the region of amino acids 701–730 and 841–860) (12, 13). The cytokine production profile of these IA-2-specific autoreactive T cells after primary stimulation was limited to TNF-α and substantial levels of IL-10 (201.2 and 745.7 pg/ml, respectively). This antiinflammatory cytokine profile matched the in situ cytokine expression in the insulitic islets and was accompanied by extremely high levels of circulating CD4+ T cells with potentially regulatory phenotype (31.4% CD4+CD25+) (14, 15).
Fig. 5.
T cell autoreactivity is restricted to IA-2 and in peripheral lymphocytes only. Shown are T cell responses to various stimuli in PBMCs (A) and intraislet lymphocytes (B) recovered from islets isolated from the explanted pancreas allograft (patient 1).
Because both the recipient and the pancreas allograft expressed HLA-A2 (0201) we further tested PBMCs for the presence of cytotoxic T cells reactive with the autoantigenic peptide of insulin B chain (HLVEALYLV) (16) or control peptide (human cytomegalovirus peptide p65, NLVPMVATV) (17): 0.03% of CD3+CD8+ T cells stained for the insulin–HLA tetramer versus 0.64% of hCMV–HLA tetramer binding cytotoxic T cells, suggesting that the degree of cytotoxic T cell autoreactivity was limited.
Islet-infiltrating leukocytes were cultured and expanded from islets isolated from the explanted pancreas allograft and tested for specificity for islet autoantigens or virus proteins. Despite good viability and strong reactivity to T cell growth factor, none of these antigens were recognized (Fig. 5B), which is in accordance with the large percentage of NK cells in the infiltrates.
Discussion
Our data provide evidence of a relation among β cell-specific enterovirus infection, insulitis, and β cell dysfunction in human type 1 diabetes, with the identification of a Coxsackie B4 virus that can persistently infect β cells, interfering with function but without triggering cell destruction. The viral infection of β cells together with the insulitic process could explain the impairment of insulin secretory function and confirms previous in vitro studies on rat and human islet cells infected with different strains of Coxsackie virus or exposed to IL-10 or to TNF-α (8, 18, 19). In addition, the expression of only these two cytokines by the diabetic islets studied is in line with the lack of β cell destruction, in the light of the findings on the protective effects of IL-10 on human islets in vitro (20) and of TNF-α on mouse islets in vivo (21), whereas IFN-γ was shown to be essential for destruction of β cells in mice (22). Furthermore, we provide a case against this insulitis being directly pathogenic to β cell, despite viral infection, because β cell insulin content and proportion of β cells per islet were similar in infected and in control islets, and no evidence of increased apoptosis was found. Our findings are corroborated by reports from other groups showing a lack of insulin response to high glucose (23), but in the presence of preserved insulin and proinsulin content, in human islet cells infected in vitro with Coxsackie B4 virus (JVB strain), and by the demonstration of a minor role of apoptosis during Coxsackie B virus infection of human islet cells in vitro. We propose that the absence of autoreactive T cells among the infiltrating leukocytes, combined with an antiinflammatory cytokine profile, could explain the lack of β cell destruction. This would be in full accordance with findings in experimental autoimmune diabetes in mice, where enterovirus was shown to be diabetogenic only in the case of a preexistent autoimmune insulitis (24), whereas the response by β cells could fundamentally determine their survival (25). In this regard it is noteworthy that Coxsackie B3 infection has been shown to suppress proinflammatory cytokines and induce IL-10 production in host cells (e.g., human monocytes) as a potential strategy to perturb the antiviral host activity leading to defective viral clearance and persistent infection (26). Further studies in mice have demonstrated that the mechanism of β cell destruction in association with Coxsackie virus infection was through bystander damage rather than molecular mimicry (27). Indeed, we have previously shown in human diabetes that any molecular homology between Coxsackie virus P2C protein and GAD65 did not lead to cross-reaction by T cell clones directed against either of the homologous sequences, despite the notion that these regions in both viral and autologous protein were subject to T cell recognition (28). Our observation that insulitis per se is not destructive to β cells in the absence of autoimmunity is also supported by studies on pancreatitis, where the vast majority of patients did not have any sign of autoimmunity or loss of β cell function, even in the presence of HLA-associated genetic predisposition to type 1 diabetes.
As far as islet cell enterovirus tropism is concerned, our results demonstrate a specific tropism to β cells in vivo, with α cells apparently being protected from enteroviral infection. The potential mechanisms responsible for this β cell specificity may include the expression of specific enterovirus receptors such as Coxsackie–adenovirus receptor and decay-accelerating factor, although no conclusive data on Coxsackie–adenovirus receptor or decay-accelerating factor expression in human islet cell subsets are available and previous studies suggested minimal levels, if any, of such expression in pancreatic islets (11). Alternatively, β cells may behave differently from α cells in terms of capacity to clear infecting enterovirus, but no data have been published to this regard.
In conclusion, this study demonstrates a correlation between β cell selective enterovirus infection and a certain pattern of insulitis. This insulitis is dominated by NK cells, lacks islet autoreactivity, is nondestructive to β cells, and nevertheless causes β cell dysfunction. Therefore, these findings imply that insulitis and autoimmunity are separate features and are both necessary for β cell destruction, whereas insulitis in the absence of autoimmunity is not β cell-destructive. Intriguingly, for cases showing viral infection of β cells and a limited degree of islet autoreactivity there was an HLA phenotype that was distinct from HLA haplotypes associated with predisposition to type 1 diabetes. Together, these findings support the hypothesis that β cell destruction requires autoimmunity with proinflammatory cytokine production, whereas viral infection by itself is not necessarily sufficient to cause this destruction. However, in a subset of type 1 diabetes patients, viral infection by itself does apparently lead to NK dominated insulitis, to β cell dysfunction, and to a deficiency in insulin secretion with consequent hyperglycemia. If true, this idea suggests that new therapeutic approaches should be considered in at least a subset of newly diagnosed patients.
Materials and Methods
Pancreata from Type 1 Diabetic Patients and Control Multiorgan Donors.
Whole pancreases were obtained from five multiorgan donors with recent-onset type 1 diabetes, one 26-year-old recipient of a whole pancreas graft, and 26 normal caucasoid multiorgan donors (12 females and 14 males, aged 14–53 years) with no family history of type 1 or type 2 diabetes. Among type 1 diabetic patients (Table 1), five were multiorgan donors (a 19-year-old male and a 14-year-old female who accidentally died 9 months and 8 months after diabetes onset, respectively; a new-onset 5-year-old boy, a new-onset 4-year-old girl, and a new-onset 15-year-old girl who died because of severe brain edema developed after diabetic ketoacidosis). One of the diabetic patients was a caucasoid type 1 diabetic woman recipient of a whole pancreas graft, which, at the time of removal (because of recurrent serious urinary tract infections), showed only partial islet function.
Immunohistochemical and Electron Microscopical Studies.
Pancreatic specimens were frozen in liquid nitrogen or formalin-fixed and paraffin-embedded for immunohistochemical investigations. The following monoclonal and polyclonal antibodies were used employing a labeled streptavidin–biotin (Dako, Glostrup, Denmark) peroxidase method: anti-insulin and anti-glucagon (Sigma, St. Louis, MO), anti-CD94 (NK cells) (Abcam, Cambridge, U.K.), anti-CD3 and anti-CD45RO (T lymphocytes), anti-CD19 (B lymphocytes), anti-CD68 (tissue macrophages), anti-IFN-α, and anti-VP-1 (Dako). The latter antibody recognizes a highly conserved epitope present in the capside protein of virtually all members of the enterovirus family (25). Because a subset of cytotoxic T cells have been shown to express CD94, double immunofluorescence with anti-CD94 and anti-CD45RO was performed to establish whether CD94-positive cells were indeed NK cells.
To determine the islet cell subset(s) infected by enterovirus, double immunofluorescence staining was performed. Anti-VP-1 reactivity was detected by using Texas red-conjugated F(ab′)2 fragment anti-mouse secondary antibody (Jackson ImmunoResearch,Newmarket, Suffolk, U.K.), and anti-insulin and anti-glucagon reactivity was detected by using, respectively, FITC-conjugated anti-guinea pig (Dako) and FITC-conjugated F(ab′)2 fragment anti-rabbit antibodies (Jackson ImmunoResearch). Sections were mounted by using Vectashield (Vector, Burlingame, CA) medium. Sections were observed by using a Sarastro 2000 confocal laser scanning microscope (Molecular Dynamics, Sunnyvale, CA). Optical sections were collected and analyzed by using ImageSpace software (Molecular Dynamics).
For electron microscopy, pancreatic samples were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) for 1 h at 4°C. After rinsing in cacodylate buffer, tissues were postfixed in 1% cacodylate-buffered osmium tetroxide for 2 h at room temperature, dehydrated, transferred to propylene oxide, and embedded in Epon-Araldite. Ultrathin sections (60–80 nm thick) were cut with a diamond knife, placed on formvar-carbon-coated copper grids (200 mesh), and stained with uranyl acetate and lead citrate.
Islet Preparation and Functional Characterization.
Purified islets were prepared by intraductal collagenase solution injection and density gradient purification (29). Insulin secretion in response to glucose and other secretagogues was assessed as previously described (20). Briefly, after a 45-min preincubation period at 3.3 mmol/liter glucose, groups of ≈30 islets of comparable size were kept at 37°C for 45 min in Krebs–Ringer bicarbonate solution (KRB), 0.5% albumin (pH 7.4), containing 3.3 mmol/liter glucose. At the end of this period, medium was completely removed and replaced with KRB containing 3.3 mmol/liter glucose, 16.7 mmol/liter glucose, 3.3 mmol/liter glucose plus 20 mmol/liter arginine, or 3.3 mmol/liter glucose plus 100 μmol/liter glibenclamide. After an additional 45-min incubation, medium was removed. Samples (500 μl) from the different media were stored at −20°C until insulin concentrations were measured by IRMA (Pantec Forniture Biomediche, Turin, Italy).
Virus Characterization.
Virus was extracted from islets of case 2 (Tuscany isolate) and subjected to whole-genome sequencing (see SI Methods for strategy and methods used).
Virus Isolation, Culture, and in Vitro Infection of Pancreatic Islets.
Virus was isolated from islets of patient 2 (Tuscany specimen) by homogenization and passaging in oral epidermoid carcinoma cells (KB cells) based on techniques previously described (30). Virus was harvested from the initial KB infection, frozen in aliquots, and thawed and amplified on KB cells only one more time before being used to infect fresh human islets. Human islets prepared from 10 independent pancreata from nondiabetic organ donors were each separately cocultured with virus-containing solution in M199 culture medium at 36°C and checked after 4 and 7 days.
For electron microscopy human islets were pelleted by centrifugation at 1,300 × g and processed as described for pancreatic samples. In addition, pelleted human islets were formalin-fixed and paraffin-embedded for immunohistochemical analysis of VP-1 expression employing the previously described immunostaining protocol.
Islet Cytokine Expression.
RT-PCR studies.
Total RNA was extracted from purified pancreatic islets with TRIzol (Gibco/BRL, Gaithersburg, MD), and RT-PCR studies were performed as previously described (20). Multiple-exon-spanning primers (31) (Life Technologies, Carlsbad, CA) specific for IL-10, IL-4, IFN-γ, and TNF-α were used. The RT-PCR signal from β-actin was analyzed as a control for the quantity of mRNA present in each sample.
Real-time PCR.
Quantitative analysis of IL-4, IL-10, IFN-γ, TGF-β, TNF-α, and β-actin mRNA expression was performed in triplicate by real-time PCR as described (32) employing TaqMan Pre-Developed Assay Reagents (Applied Biosystems, Foster City, CA) and the ABI PRISM 7700 Sequence Detection System (Applied Biosystems). Results are expressed as percentage of β-actin mRNA expression.
ELISAs.
IL-4, IL-10, IFNγ, and TNF-α concentrations were determined in supernatants of cultured pancreatic islets by commercially available ELISA kits (Endogen, Rockford, IL). TGFβ concentrations were determined by using a commercially available TGF-β Quantikine kit (R & D Systems, Abingdon, U.K.). Optical densities were measured on a Bio-Rad ELISA reader at a wavelength of 450 nm. Data were analyzed against the linear portion of the generated standard curve.
Functional Studies on Peripheral Blood Lymphocytes and Islet-Infiltrating Cells.
PBMCs were isolated by Ficoll gradient centrifugation and resuspended in complete IMDM supplemented with 2 mmol/liter glutamine, 100 units/ml penicillin, 100 units/ml streptomycin, and 10% pooled human AB serum. PBMCs were cultured for 5 days in a lymphocyte stimulation assay (33) in the absence or presence of the following antigens: 10 μg/ml rGAD65 (Diamyd, Stockholm, Sweden), 25 μg/ml rInsulin (Sigma), 10 μg/ml rIA-2 (generously provided by M. R. Christie, Guy's King's College and St. Thomas' School of Medicine, London, U.K.) (12), 1.5 Lf/ml purified tetanus toxoid (National Institute of Health, Bilthoven, The Netherlands), or 10% IL-2 (Lymphocult-T; Biotest, Solihull, U.K.) in 96-well round-bottom plates (Costar, Cambridge, MA). After 4 days, cultures were pulsed with [3H]thymidine (0.5 μCi per well) for the final 18 h of culture, after which [3H]thymidine incorporation was measured by liquid scintillation counting. Results are expressed as means ± SD of triplicate wells. Before [3H]thymidine addition, 4-day culture supernatant was collected for autoantigen-specific cytokine analysis via the human Th1/Th2 cytometric bead array kit according to the manufacturer's protocol (Becton Dickinson Biosciences, Franklin Lakes, NJ). For evaluation of CD25 expression on PBMCs, cells were stained for 30 min at 4°C with CD4-APC and CD25-FITC (Becton Dickinson Biosciences) and subsequently analyzed on a FACSCalibur.
Intraislet lymphocytes were cultured from purified islets by growing 1 × 105 cells in complete IMDM supplemented with 10% pooled AB serum, 10% Lymphocult-T (as source of IL-2), and 1% PHA (Murex Biotech, Dartford, U.K.) in the presence of 2 × 105 2,000-rad irradiated autologous PBMCs. Fresh Lymphocult-T-containing medium was added every 2–3 days. After 7 days of culture, intraislet lymphocytes were restimulated with 1 × 106 feeder cells (3,000-rad irradiated PBMCs from HLA-matched donors) in the presence of 1% PHA. Cells were cultured from day 2 until day 6 in Lymphocult-T-containing IMDM. After this round of expansion, 2 × 104 intraislet lymphocytes and 1 × 105 HLA-matched PBMCs (2,000-rad irradiated) were tested in a proliferation assay in a 96-well flat-bottom plate (Costar) in the absence or presence of the same antigens as described for PBMCs. After 3 days, cultures were pulsed with [3H]thymidine (0.5 μCi per well) for the final 18 h of culture. Results are expressed as means ± SD of triplicate wells.
Supplementary Material
Acknowledgments
We thank Dr. A. Scipioni for immunohistochemical studies, G. Duinkerken for the autoreactive T cell studies, Drs. F. Maggi and M. Giorgi for virus culture and in vitro islet infection, and Dr. J. Lakey (Clinical Islet Transplant Center, University of Alberta) for providing paraffin sections of 16 control pancreata for this study. We give special thanks to Dr. Silvia Guidotti and Dr. Alessandro Muzzi of the Cellular Microbiology and Bioinformatic Unit of Novartis Vaccines for their invaluable help in sequence retrieval and phylogenetic analyses. This work was supported by grants from the Juvenile Diabetes Research Foundation International, the European Foundation for the Study of Diabetes (a European Foundation for the Study of Diabetes/Pfizer research grant), the Italian Ministry of Health, the Dutch Diabetes Research Foundation, the Promoter Foundation ONLUS, and the Canadian Institutes of Health Research.
Abbreviations
- NK
natural killer
- PBMC
peripheral blood mononuclear cell.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequence reported in this paper for the Coxsackie B4 genome (Tuscany isolate) has been deposited in the GenBank database (accession no. DQ480420).
This article contains supporting information online at www.pnas.org/cgi/content/full/0700442104/DC1.
References
- 1.Gale EA. Diabetes. 2002;51:3353–3361. doi: 10.2337/diabetes.51.12.3353. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson MA, Eisenbarth GS. Lancet. 2001;358:221–229. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 3.von Herrath MG. Curr Top Microbiol Immunol. 2002;263:145–175. doi: 10.1007/978-3-642-56055-2_8. [DOI] [PubMed] [Google Scholar]
- 4.Hyoty H, Hiltunen M, Lonnrot M. Clin Diagn Virol. 1998;9:77–84. doi: 10.1016/s0928-0197(98)00007-5. [DOI] [PubMed] [Google Scholar]
- 5.Yin H, Berg AK, Tuvemo T, Frisk G. Diabetes. 2002;51:1964–1971. doi: 10.2337/diabetes.51.6.1964. [DOI] [PubMed] [Google Scholar]
- 6.Yoon JW, Austin M, Onodera T, Notkins AL. N Engl J Med. 1979;300:1173–1179. doi: 10.1056/NEJM197905243002102. [DOI] [PubMed] [Google Scholar]
- 7.See DM, Tilles JG. J Infect Dis. 1995;171:1131–1138. doi: 10.1093/infdis/171.5.1131. [DOI] [PubMed] [Google Scholar]
- 8.Roivainen M, Ylipaasto P, Savolainen C, Galama J, Hovi T, Otonkoski T. Diabetologia. 2002;45:693–702. doi: 10.1007/s00125-002-0805-x. [DOI] [PubMed] [Google Scholar]
- 9.Hiemstra HS, Schloot NC, van Veelen PA, Willemen SJM, Franken KLMC, van Rood JJ, de Vries RRP, Chaudhuri A, Behan PO, Drijfhout JW, Roep BO. Proc Natl Acad Sci USA. 2001;98:3988–3991. doi: 10.1073/pnas.071050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roep BO, Hiemstra HS, Schloot NC, De Vries RR, Chaudhuri A, Behan PO, Drijfhout JW. Ann NY Acad Sci. 2002;958:163–165. [PubMed] [Google Scholar]
- 11.Ylipaasto P, Klingel K, Lindberg AM, Otonkoski T, Kandolf R, Hovi T, Roivainen M. Diabetologia. 2004;47:225–239. doi: 10.1007/s00125-003-1297-z. [DOI] [PubMed] [Google Scholar]
- 12.Hawkes CJ, Schloot NC, Marks J, Willemen SJM, Drijfhout JW, Mayer EK, Christie MR, Roep BO. Diabetes. 2000;49:356–366. doi: 10.2337/diabetes.49.3.356. [DOI] [PubMed] [Google Scholar]
- 13.Arif S, Tree TI, Astill TP, Tremble JM, Bishop AJ, Dayan CM, Roep BO, Peakman M. J Clin Invest. 2004;113:451–463. doi: 10.1172/JCI19585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. J Immunol. 2002;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 15.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Diabetes. 2005;54:92–99. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 16.Pinkse GG, Tysma OH, Bergen CA, Kester MG, Ossendorp F, van Veelen PA, Keymeulen B, Pipeleers D, Drijfhout JW, Roep BO. Proc Natl Acad Sci USA. 2005;102:18425–18430. doi: 10.1073/pnas.0508621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huurman VAL, Kalpoe JS, van de Linde P, Vaessen N, Ringers J, Kroes ACM, Roep BO, de Fijter JW. Diabetes Care. 2006;29:842–847. doi: 10.2337/diacare.29.04.06.dc05-1647. [DOI] [PubMed] [Google Scholar]
- 18.Laffranchi R, Spinas GA. Eur J Endocrinol. 1996;135:374–378. doi: 10.1530/eje.0.1350374. [DOI] [PubMed] [Google Scholar]
- 19.Zhang S, Kim KH. FEBS Lett. 1995;377:237–239. doi: 10.1016/0014-5793(95)01272-9. [DOI] [PubMed] [Google Scholar]
- 20.Marselli L, Dotta F, Piro S, Santangelo C, Masini M, Lupi R, Realacci M, Del Guerra S, Mosca F, Boggi U, et al. J Clin Endocrinol Metab. 2001;86:4974–4978. doi: 10.1210/jcem.86.10.7938. [DOI] [PubMed] [Google Scholar]
- 21.Christen U, Wolfe T, Mohrle U, Hughes AC, Rodrigo E, Green EA, Flavell RA, von Herrath MG. J Immunol. 2001;166:7023–7032. doi: 10.4049/jimmunol.166.12.7023. [DOI] [PubMed] [Google Scholar]
- 22.von Herrath MG, Oldstone MBA. J Exp Med. 1997;185:531–539. doi: 10.1084/jem.185.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin H, Berg AK, Westman J, Hellerstrom C, Frisk G. J Med Virol. 2002;68:544–557. doi: 10.1002/jmv.10236. [DOI] [PubMed] [Google Scholar]
- 24.Serreze DV, Ottendorfer EW, Ellis TM, Gauntt CJ, Atkinson MA. Diabetes. 2000;49:708–711. doi: 10.2337/diabetes.49.5.708. [DOI] [PubMed] [Google Scholar]
- 25.Flodstrom M, Maday A, Balakrishna D, Cleary MM, Yoshimura A, Sarvetnick N. Nat Immunol. 2002;3:373–382. doi: 10.1038/ni771. [DOI] [PubMed] [Google Scholar]
- 26.Hofmann P, Schmidtke M, Stelzner A, Gemsa D. J Med Virol. 2001;64:487–498. doi: 10.1002/jmv.1076. [DOI] [PubMed] [Google Scholar]
- 27.Horwitz MS, Bradley LM, Harbertson J, Krahl T, Lee J, Sarvetnick N. Nat Med. 1998;4:781–785. doi: 10.1038/nm0798-781. [DOI] [PubMed] [Google Scholar]
- 28.Schloot NC, Willemen SJ, Duinkerken G, Drijfhout JW, De Vries RR, Roep BO. Hum Immunol. 2001;62:299–309. doi: 10.1016/s0198-8859(01)00223-3. [DOI] [PubMed] [Google Scholar]
- 29.Lupi R, Dotta F, Marselli L, Del Guerra S, Masini M, Santangelo C, Patane G, Boggi U, Piro S, Anello M, et al. Diabetes. 2002;51:1437–1442. doi: 10.2337/diabetes.51.5.1437. [DOI] [PubMed] [Google Scholar]
- 30.Morrica A, Giorgi M, Maggi F, Fornai C, Vatteroni ML, Marchi S, Ricchiuti A, Antonelli G, Pistello M, Bendinelli M. J Virol Methods. 1999;77:207–215. doi: 10.1016/s0166-0934(98)00155-4. [DOI] [PubMed] [Google Scholar]
- 31.Habibi GR, Khamesipour A, McMaster WR, Mahboudi F. Scand J Immunol. 2001;54:414–420. doi: 10.1046/j.1365-3083.2001.00990.x. [DOI] [PubMed] [Google Scholar]
- 32.Wang T, Brown MJ. Anal Biochem. 1999;269:198–201. doi: 10.1006/abio.1999.4022. [DOI] [PubMed] [Google Scholar]
- 33.Roep BO, Kallan AA, Duinkerken G, Arden SD, Hutton JC, Bruining GJ, de Vries RR. Diabetes. 1995;44:278–283. doi: 10.2337/diab.44.3.278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.