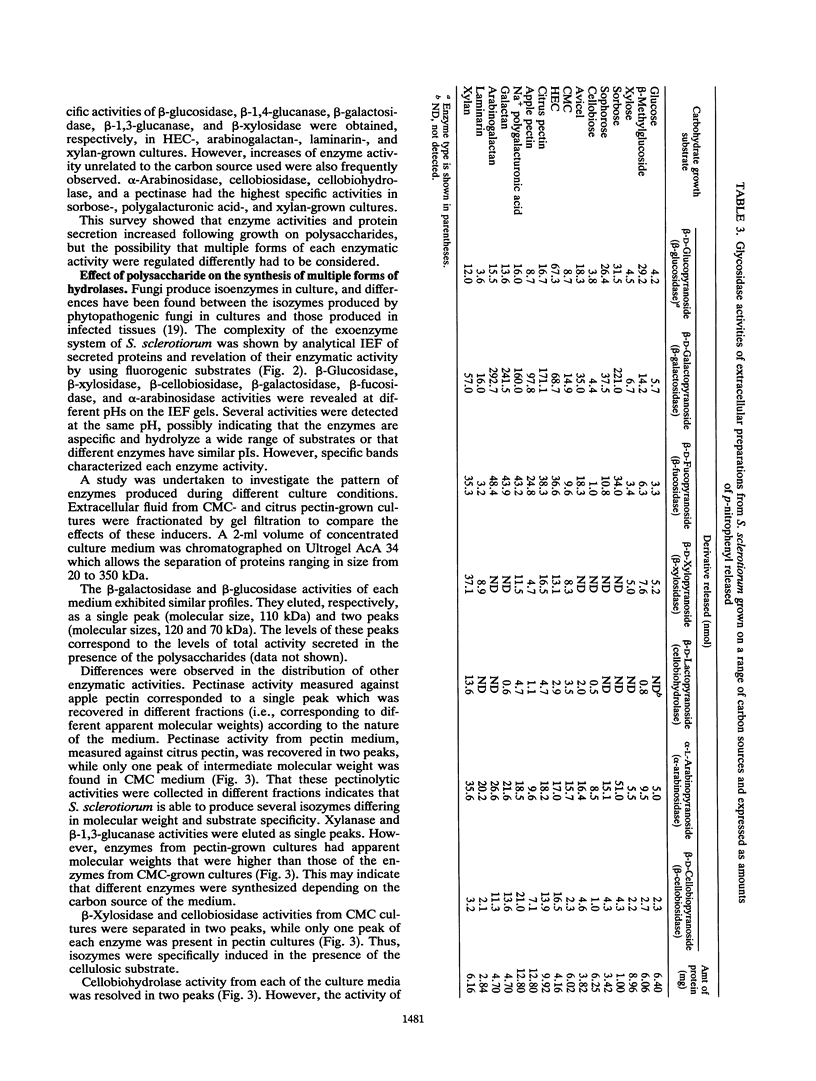
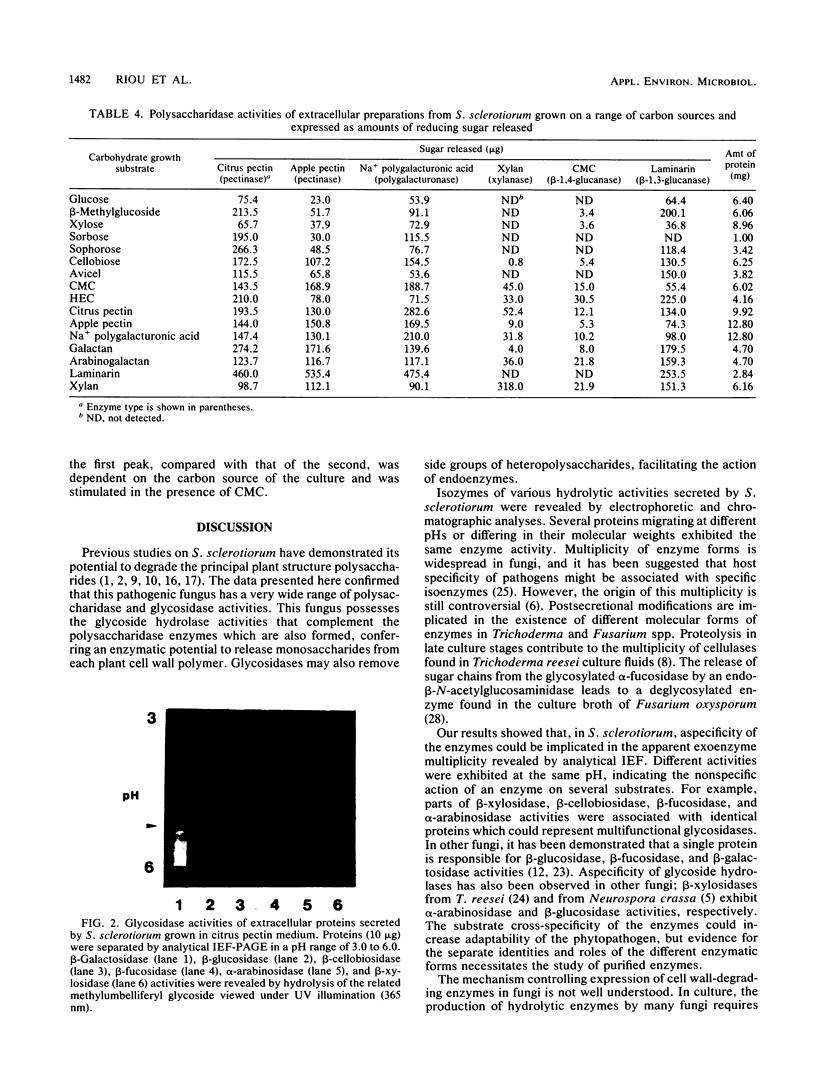
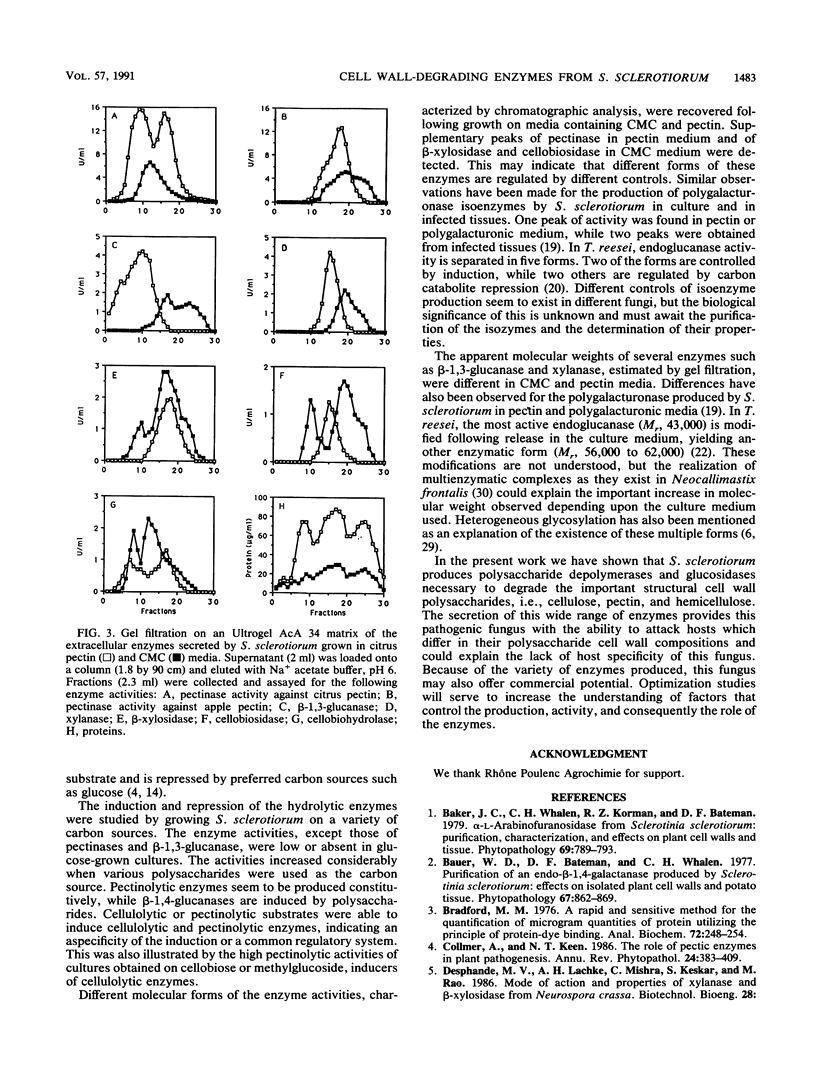
Abstract
The range of polysaccharide-degrading enzymes and glycosidases formed by the phytopathogenic fungus Sclerotinia sclerotiorum was monitored following growth on 16 carbohydrate substrates. Endo- and exoenzymes capable of degrading cellulosic, hemicellulosic, and pectinolytic polysaccharides were secreted. Pectinolytic activities were produced constitutively on all of the substrates tested. Cellulolytic enzymes were not induced in simple sugar (i.e., glucose or xylose) media. Polysaccharide growth substrates and cellulase inducers increased all of the enzyme activities tested. Gel filtration analysis revealed the appearance of new molecular forms of pectinase, β-xylosidase, and cellobiosidase during induction on pectin and carboxymethyl cellulose media.
Full text
PDF
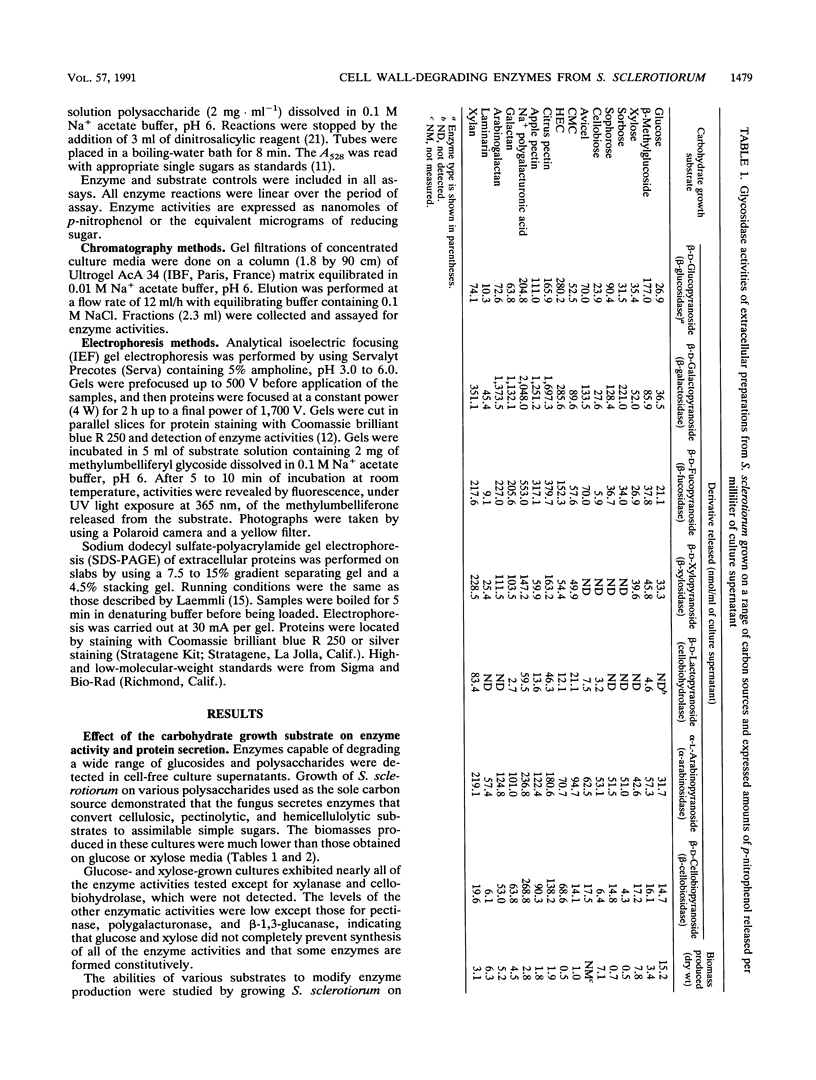
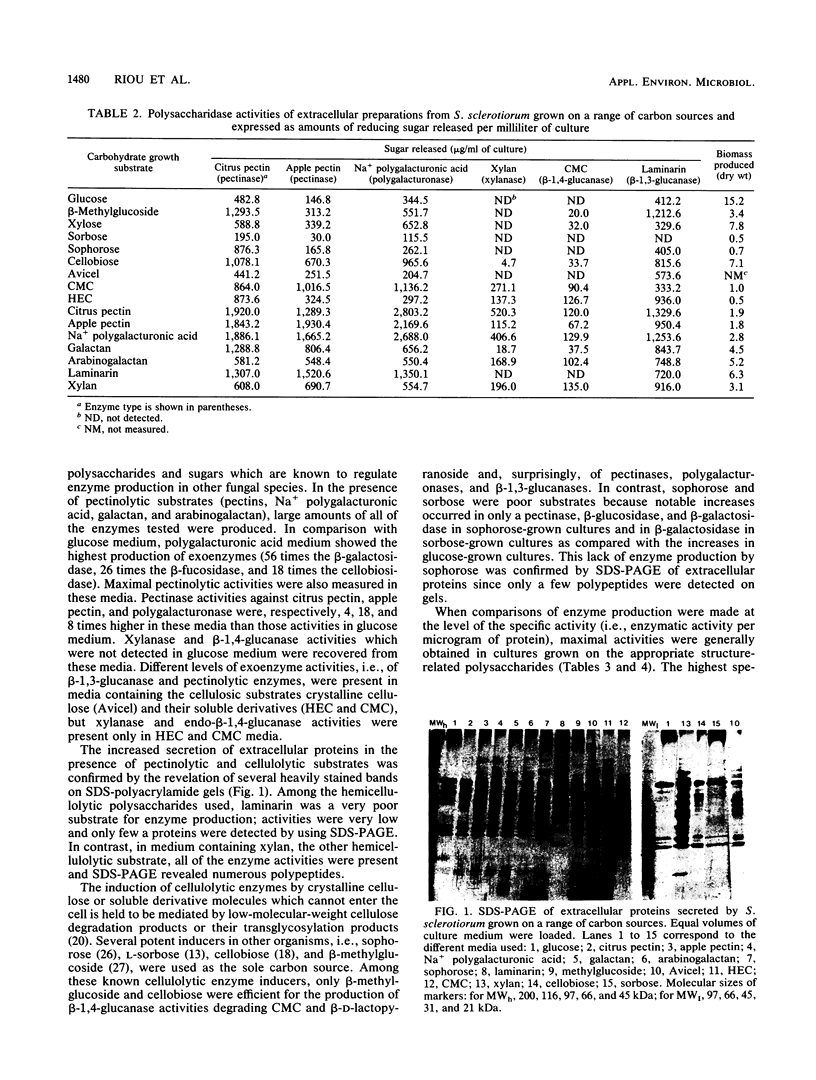

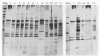
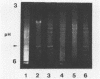
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Hebraud M., Fevre M. Purification and Characterization of an Aspecific Glycoside Hydrolase from the Anaerobic Ruminal Fungus Neocallimastix frontalis. Appl Environ Microbiol. 1990 Oct;56(10):3164–3169. doi: 10.1128/aem.56.10.3164-3169.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MANDELS M., REESE E. T. Induction of cellulase in fungi by cellobiose. J Bacteriol. 1960 Jun;79:816–826. doi: 10.1128/jb.79.6.816-826.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner R., Gruber F., Kubicek C. P. Differential regulation of synthesis of multiple forms of specific endoglucanases by Trichoderma reesei QM9414. J Bacteriol. 1988 Aug;170(8):3689–3693. doi: 10.1128/jb.170.8.3689-3693.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niku-Paavola M. L., Lappalainen A., Enari T. M., Nummi M. A new appraisal of the endoglucanases of the fungus Trichoderma reesei. Biochem J. 1985 Oct 1;231(1):75–81. doi: 10.1042/bj2310075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta R. M., Terenzi H. F., Jorge J. A. Beta-D-glycosidase activities of Humicola grisea: biochemical and kinetic characterization of a multifunctional enzyme. Biochim Biophys Acta. 1990 Mar 26;1033(3):243–249. doi: 10.1016/0304-4165(90)90127-i. [DOI] [PubMed] [Google Scholar]
- Starr M. P., Chatterjee A. K. The genus Erwinia: enterobacteria pathogenic to plants and animals. Annu Rev Microbiol. 1972;26:389–426. doi: 10.1146/annurev.mi.26.100172.002133. [DOI] [PubMed] [Google Scholar]
- Sternberg D., Mandels G. R. Induction of cellulolytic enzymes in Trichoderma reesei by sophorose. J Bacteriol. 1979 Sep;139(3):761–769. doi: 10.1128/jb.139.3.761-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji Y., Yamamoto K., Tochikura T. Formation of deglycosylated alpha-L-fucosidase by endo-beta-N-acetylglucosaminidase in Fusarium oxysporum. Appl Environ Microbiol. 1990 Apr;56(4):928–933. doi: 10.1128/aem.56.4.928-933.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willick G. E., Seligy V. L. Multiplicity in cellulases of Schizophyllum commune. Derivation partly from heterogeneity in transcription and glycosylation. Eur J Biochem. 1985 Aug 15;151(1):89–96. doi: 10.1111/j.1432-1033.1985.tb09072.x. [DOI] [PubMed] [Google Scholar]