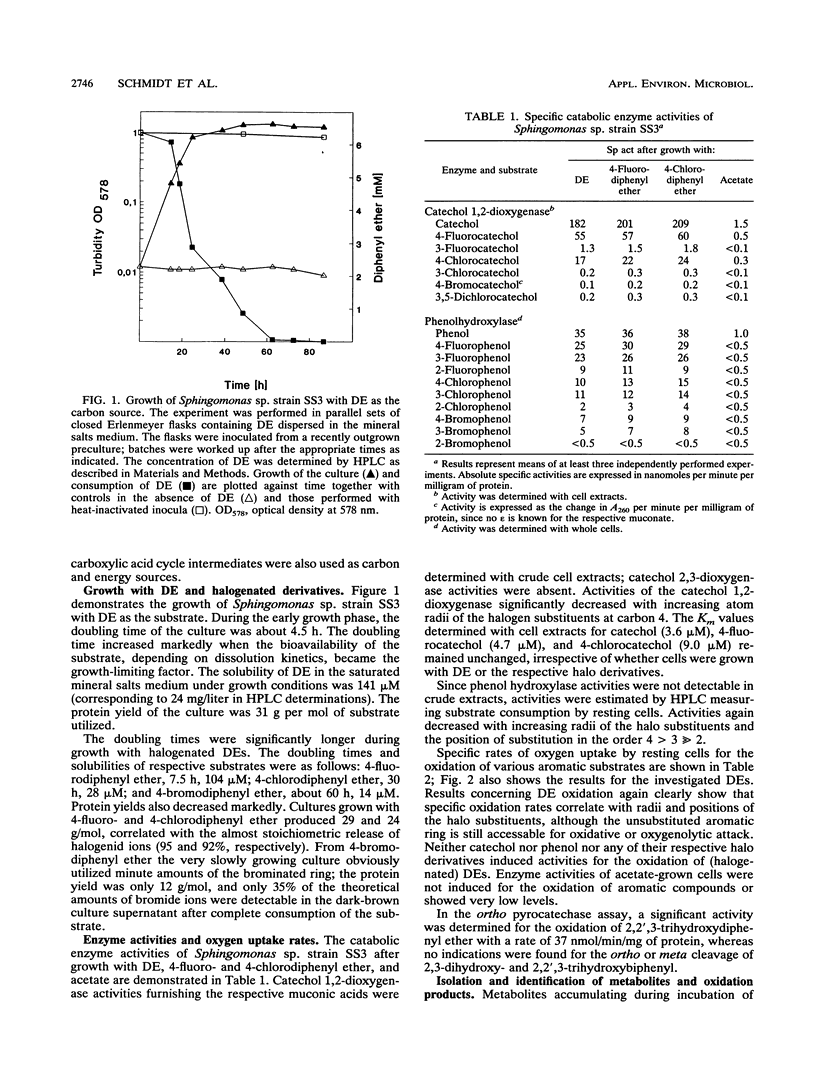
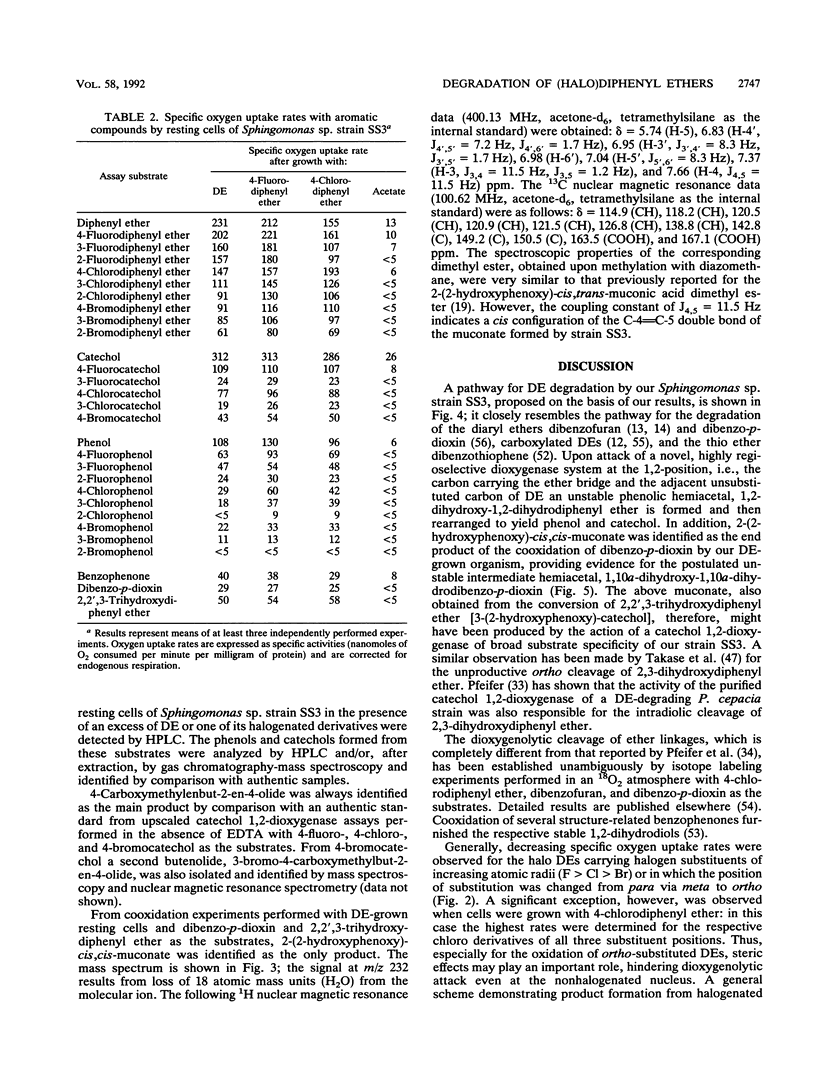
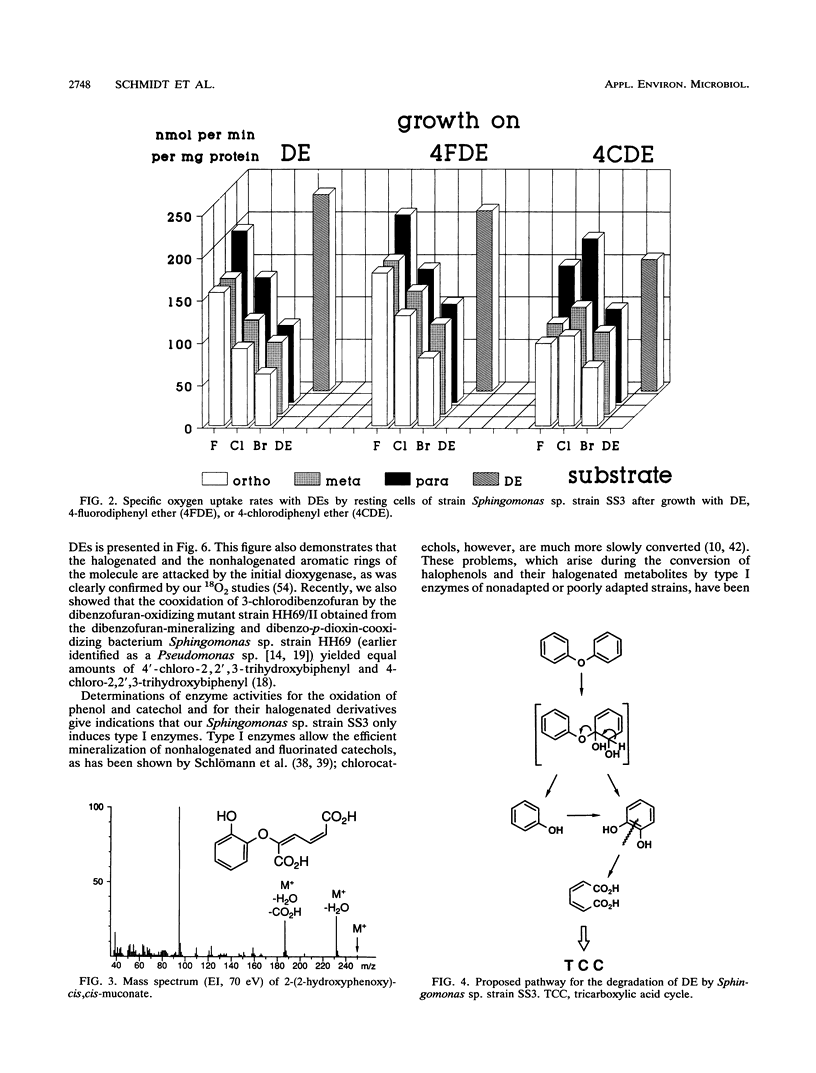
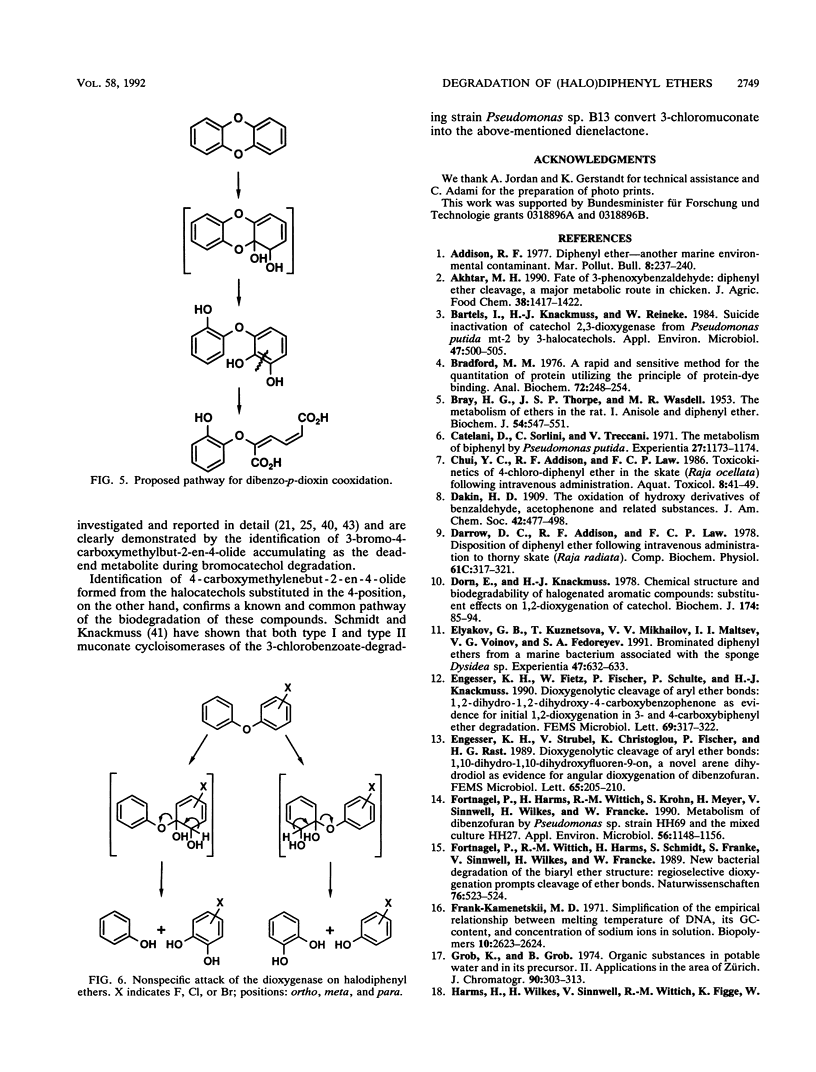
Abstract
The bacterium Sphingomonas sp. strain SS3, which utilizes diphenyl ether and its 4-fluoro, 4-chloro, and (to a considerably lesser extent) 4-bromo derivatives as sole sources of carbon and energy, was enriched from soil samples of an industrial waste deposit. The bacterium showed cometabolic activities toward all other isomeric monohalogenated diphenyl ethers. During diphenyl ether degradation in batch culture experiments, phenol and catechol were produced as intermediates which were then channeled into the 3-oxoadipate pathway. The initial step in the degradation follows the recently discovered mechanism of 1,2-dioxygenation, which yields unstable phenolic hemiacetals from diphenyl ether structures. Oxidation of the structure-related dibenzo-p-dioxin yielded 2-(2-hydroxyphenoxy)-muconate upon ortho cleavage of the intermediate 2,2',3-trihydroxydiphenyl ether. Formation of phenol, catechol, halophenol, and halocatechol from the conversion of monohalogenated diphenyl ethers gives evidence for a nonspecific attack of the dioxygenating enzyme system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAY H. G., JAMES S. P., THORPE W. V., WASDELL M. R. The metabolism of ethers in the rabbit. I. Anisole and diphenyl ether. Biochem J. 1953 Jul;54(4):547–551. [PMC free article] [PubMed] [Google Scholar]
- Bartels I., Knackmuss H. J., Reineke W. Suicide Inactivation of Catechol 2,3-Dioxygenase from Pseudomonas putida mt-2 by 3-Halocatechols. Appl Environ Microbiol. 1984 Mar;47(3):500–505. doi: 10.1128/aem.47.3.500-505.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Catelani D., Sorlini C., Treccani V. The metabolism of biphenyl by Pseudomonas putida. Experientia. 1971 Oct 15;27(10):1173–1174. doi: 10.1007/BF02286908. [DOI] [PubMed] [Google Scholar]
- Darrow D. C., Addison R. F. Disposition of diphenyl ether following intravenous administration to thorny skate (Raja radiata). Comp Biochem Physiol C. 1978;61 100(2):317–321. doi: 10.1016/0306-4492(78)90061-8. [DOI] [PubMed] [Google Scholar]
- Dorn E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Substituent effects on 1,2-dioxygenation of catechol. Biochem J. 1978 Jul 15;174(1):85–94. doi: 10.1042/bj1740085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engesser K. H., Fietz W., Fischer P., Schulte P., Knackmuss H. J. Dioxygenolytic cleavage of aryl ether bonds: 1,2-dihydro-1,2-dihydroxy-4-carboxybenzophenone as evidence for initial 1,2-dioxygenation in 3- and 4-carboxy biphenyl ether degradation. FEMS Microbiol Lett. 1990 Jun 1;57(3):317–321. doi: 10.1016/0378-1097(90)90087-7. [DOI] [PubMed] [Google Scholar]
- Engesser K. H., Strubel V., Christoglou K., Fischer P., Rast H. G. Dioxygenolytic cleavage of aryl ether bonds: 1,10-dihydro-1,10-dihydroxyfluoren-9-one, a novel arene dihydrodiol as evidence for angular dioxygenation of dibenzofuran. FEMS Microbiol Lett. 1989 Nov;53(1-2):205–209. doi: 10.1016/0378-1097(89)90392-3. [DOI] [PubMed] [Google Scholar]
- Fortnagel P., Harms H., Wittich R. M., Krohn S., Meyer H., Sinnwell V., Wilkes H., Francke W. Metabolism of Dibenzofuran by Pseudomonas sp. Strain HH69 and the Mixed Culture HH27. Appl Environ Microbiol. 1990 Apr;56(4):1148–1156. doi: 10.1128/aem.56.4.1148-1156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank-Kamenetskii F. Simplification of the empirical relationship between melting temperature of DNA, its GC content and concentration of sodium ions in solution. Biopolymers. 1971;10(12):2623–2624. doi: 10.1002/bip.360101223. [DOI] [PubMed] [Google Scholar]
- Grob K., Grob G. Organic substances in potable water and in its precursor. II. Applications in the area of Zürich. J Chromatogr. 1974 Apr 10;90(2):303–313. doi: 10.1016/s0021-9673(00)92532-9. [DOI] [PubMed] [Google Scholar]
- Harms H., Wittich R. M., Sinnwell V., Meyer H., Fortnagel P., Francke W. Transformation of Dibenzo-p-Dioxin by Pseudomonas sp. Strain HH69. Appl Environ Microbiol. 1990 Apr;56(4):1157–1159. doi: 10.1128/aem.56.4.1157-1159.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke D., Fritsche W. Dechlorierung von 4-Chlorphenol nach extradioler Ringspaltung durch Pseudomonas putida. Z Allg Mikrobiol. 1979;19(2):139–141. doi: 10.1002/jobm.3630190210. [DOI] [PubMed] [Google Scholar]
- Klecka G. M., Gibson D. T. Inhibition of catechol 2,3-dioxygenase from Pseudomonas putida by 3-chlorocatechol. Appl Environ Microbiol. 1981 May;41(5):1159–1165. doi: 10.1128/aem.41.5.1159-1165.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackmuss H. J., Hellwig M. Utilization and cooxidation of chlorinated phenols by Pseudomonas sp. B 13. Arch Microbiol. 1978 Apr 27;117(1):1–7. doi: 10.1007/BF00689343. [DOI] [PubMed] [Google Scholar]
- Liaw H. J., Srinivasan V. R. Expression of an Erwinia sp. gene encoding diphenyl ether cleavage in Escherichia coli and an isolated Acinetobacter strain PE7. Appl Microbiol Biotechnol. 1990 Mar;32(6):686–689. doi: 10.1007/BF00164740. [DOI] [PubMed] [Google Scholar]
- Liaw H. J., Srinivasan V. R. Molecular cloning and expression of an Erwinia sp. gene encoding diphenyl ether cleavage in Escherichia coli. Appl Environ Microbiol. 1989 Sep;55(9):2220–2225. doi: 10.1128/aem.55.9.2220-2225.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T., Yamagishi T., Matsumoto M. Residues of 4-chloro-1-(2,4-dichlorophenoxy)-2-methoxybenzene(triclosan methyl) in aquatic biota. Bull Environ Contam Toxicol. 1984 Feb;32(2):227–232. doi: 10.1007/BF01607490. [DOI] [PubMed] [Google Scholar]
- Sander P., Wittich R. M., Fortnagel P., Wilkes H., Francke W. Degradation of 1,2,4-trichloro- and 1,2,4,5-tetrachlorobenzene by pseudomonas strains. Appl Environ Microbiol. 1991 May;57(5):1430–1440. doi: 10.1128/aem.57.5.1430-1440.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlömann M., Fischer P., Schmidt E., Knackmuss H. J. Enzymatic formation, stability, and spontaneous reactions of 4-fluoromuconolactone, a metabolite of the bacterial degradation of 4-fluorobenzoate. J Bacteriol. 1990 Sep;172(9):5119–5129. doi: 10.1128/jb.172.9.5119-5129.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlömann M., Schmidt E., Knackmuss H. J. Different types of dienelactone hydrolase in 4-fluorobenzoate-utilizing bacteria. J Bacteriol. 1990 Sep;172(9):5112–5118. doi: 10.1128/jb.172.9.5112-5118.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E., Hellwig M., Knackmuss H. J. Degradation of chlorophenols by a defined mixed microbial community. Appl Environ Microbiol. 1983 Nov;46(5):1038–1044. doi: 10.1128/aem.46.5.1038-1044.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Conversion of chlorinated muconic acids into maleoylacetic acid. Biochem J. 1980 Oct 15;192(1):339–347. doi: 10.1042/bj1920339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E., Remberg G., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Halogenated muconic acids as intermediates. Biochem J. 1980 Oct 15;192(1):331–337. doi: 10.1042/bj1920331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwien U., Schmidt E. Improved degradation of monochlorophenols by a constructed strain. Appl Environ Microbiol. 1982 Jul;44(1):33–39. doi: 10.1128/aem.44.1.33-39.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strubel V., Engesser K. H., Fischer P., Knackmuss H. J. 3-(2-hydroxyphenyl)catechol as substrate for proximal meta ring cleavage in dibenzofuran degradation by Brevibacterium sp. strain DPO 1361. J Bacteriol. 1991 Mar;173(6):1932–1937. doi: 10.1128/jb.173.6.1932-1937.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulp M. T., Sundström G., Martron L. B., Hutzinger O. Metabolism of chlorodiphenyl ethers and Irgasan DP 300. Xenobiotica. 1979 Feb;9(2):65–77. doi: 10.3109/00498257909038708. [DOI] [PubMed] [Google Scholar]
- Wittich R. M., Wilkes H., Sinnwell V., Francke W., Fortnagel P. Metabolism of dibenzo-p-dioxin by Sphingomonas sp. strain RW1. Appl Environ Microbiol. 1992 Mar;58(3):1005–1010. doi: 10.1128/aem.58.3.1005-1010.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuuchi E., Yano I., Oyaizu H., Hashimoto Y., Ezaki T., Yamamoto H. Proposals of Sphingomonas paucimobilis gen. nov. and comb. nov., Sphingomonas parapaucimobilis sp. nov., Sphingomonas yanoikuyae sp. nov., Sphingomonas adhaesiva sp. nov., Sphingomonas capsulata comb. nov., and two genospecies of the genus Sphingomonas. Microbiol Immunol. 1990;34(2):99–119. doi: 10.1111/j.1348-0421.1990.tb00996.x. [DOI] [PubMed] [Google Scholar]


