Abstract
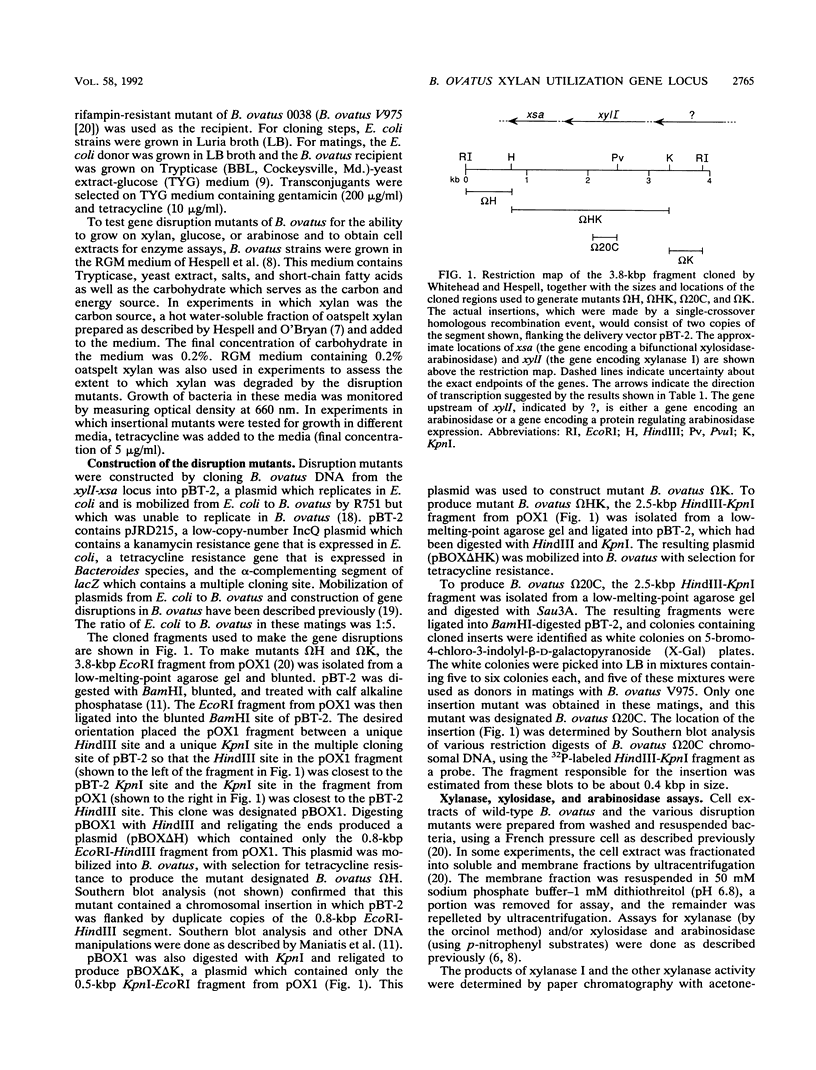
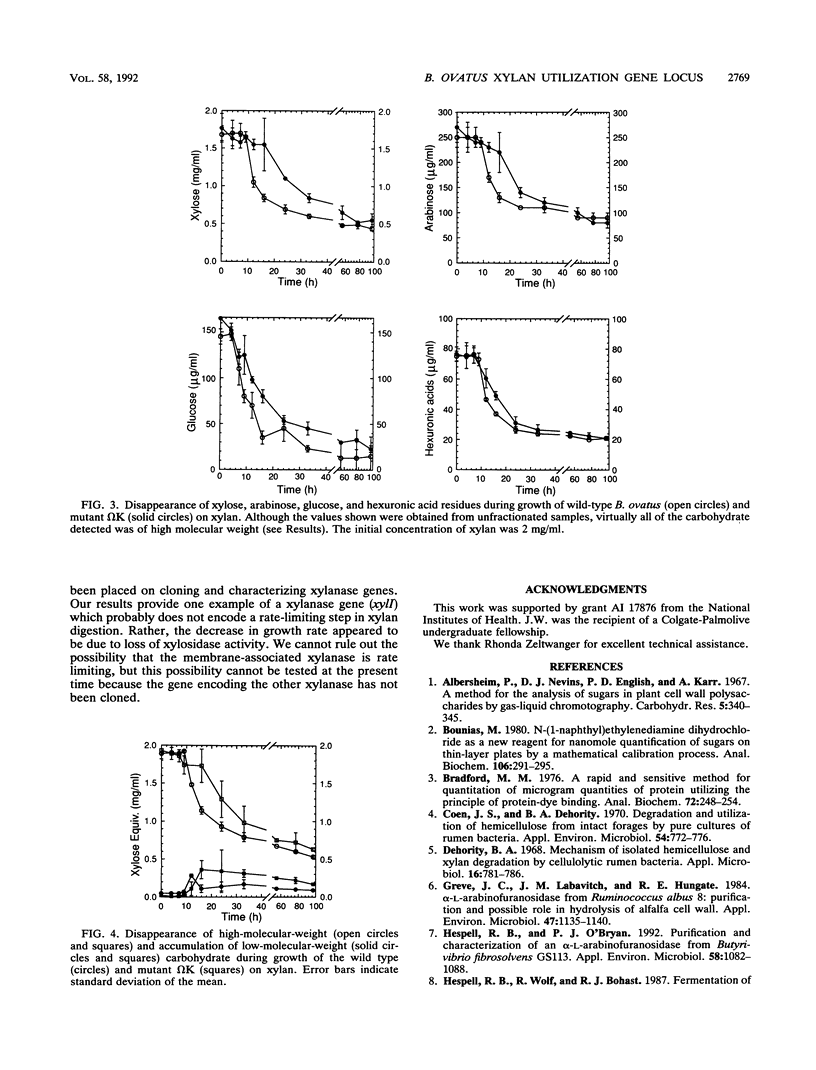
Bacteroides ovatus, a gram-negative obligate anaerobe found in the human colon, can utilize xylan as a sole source of carbohydrate. Previously, a 3.8-kbp segment of B. ovatus chromosomal DNA, which contained genes encoding a xylanase (xylI) and a bifunctional xylosidase-arabinosidase (xsa), was cloned, and expression of the two genes was studied in Escherichia coli (T. Whitehead and R. Hespell, J. Bacteriol. 172:2408-2412, 1990). In the present study, we have used segments of the cloned region to construct insertional disruptions in the B. ovatus chromosomal locus containing these two genes. Analysis of these insertional mutants demonstrated that (i) xylI and xsa are probably part of the same operon, with xylI upstream of xsa, (ii) the true B. ovatus promoter was not cloned on the 3.5-kbp DNA fragment which expressed xylanase and xylosidase in E. coli, (iii) there is at least one gene upstream of xylI which could encode an arabinosidase, and (iv) xylosidase rather than xylanase may be a rate-limiting step in xylan utilization. Insertional mutations in the xylI-xsa locus reduced the rate of growth on xylan, but the concentration of residual sugars at the end of growth was the same as that with the wild type. Thus, a slower rate of growth on xylan was not accompanied by less extensive digestion of xylan. Mutants in which xylI had been disrupted still expressed some xylanase activity. This second activity was associated with membranes and produced xylose from xylan, whereas the xylI gene product partitioned primarily with the soluble fraction and produced xylobiose from xylan.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bounias M. N-(1-naphthyl)ethylenediamine dihydrochloride as a new reagent for nanomole quantification of sugars on thin-layer plates by a mathematical calibration process. Anal Biochem. 1980 Aug;106(2):291–295. doi: 10.1016/0003-2697(80)90523-0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Dehority B. A. Mechanism of isolated hemicellulose and xylan degradation by cellulolytic rumen bacteria. Appl Microbiol. 1968 May;16(5):781–786. doi: 10.1128/am.16.5.781-786.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve L. C., Labavitch J. M., Hungate R. E. alpha-L-arabinofuranosidase from Ruminococcus albus 8: purification and possible role in hydrolysis of alfalfa cell wall. Appl Environ Microbiol. 1984 May;47(5):1135–1140. doi: 10.1128/aem.47.5.1135-1140.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hespell R. B., O'bryan P. J. Purification and Characterization of an alpha-l-Arabinofuranosidase from Butyrivibrio fibrisolvens GS113. Appl Environ Microbiol. 1992 Apr;58(4):1082–1088. doi: 10.1128/aem.58.4.1082-1088.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., Gherardini F., O'Brien M. Utilization of xylan by two species of human colonic Bacteroides. Appl Environ Microbiol. 1981 Apr;41(4):1065–1068. doi: 10.1128/aem.41.4.1065-1068.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salyers A. A., Vercellotti J. R., West S. E., Wilkins T. D. Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl Environ Microbiol. 1977 Feb;33(2):319–322. doi: 10.1128/aem.33.2.319-322.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah H. N., Collins D. M. Prevotella, a new genus to include Bacteroides melaninogenicus and related species formerly classified in the genus Bacteroides. Int J Syst Bacteriol. 1990 Apr;40(2):205–208. doi: 10.1099/00207713-40-2-205. [DOI] [PubMed] [Google Scholar]
- Valentine P. J., Arnold P., Salyers A. A. Cloning and partial characterization of two chromosomal loci from Bacteroides ovatus that contain genes essential for growth on guar gum. Appl Environ Microbiol. 1992 May;58(5):1541–1548. doi: 10.1128/aem.58.5.1541-1548.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead T. R., Hespell R. B. The genes for three xylan-degrading activities from Bacteroides ovatus are clustered in a 3.8-kilobase region. J Bacteriol. 1990 May;172(5):2408–2412. doi: 10.1128/jb.172.5.2408-2412.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]