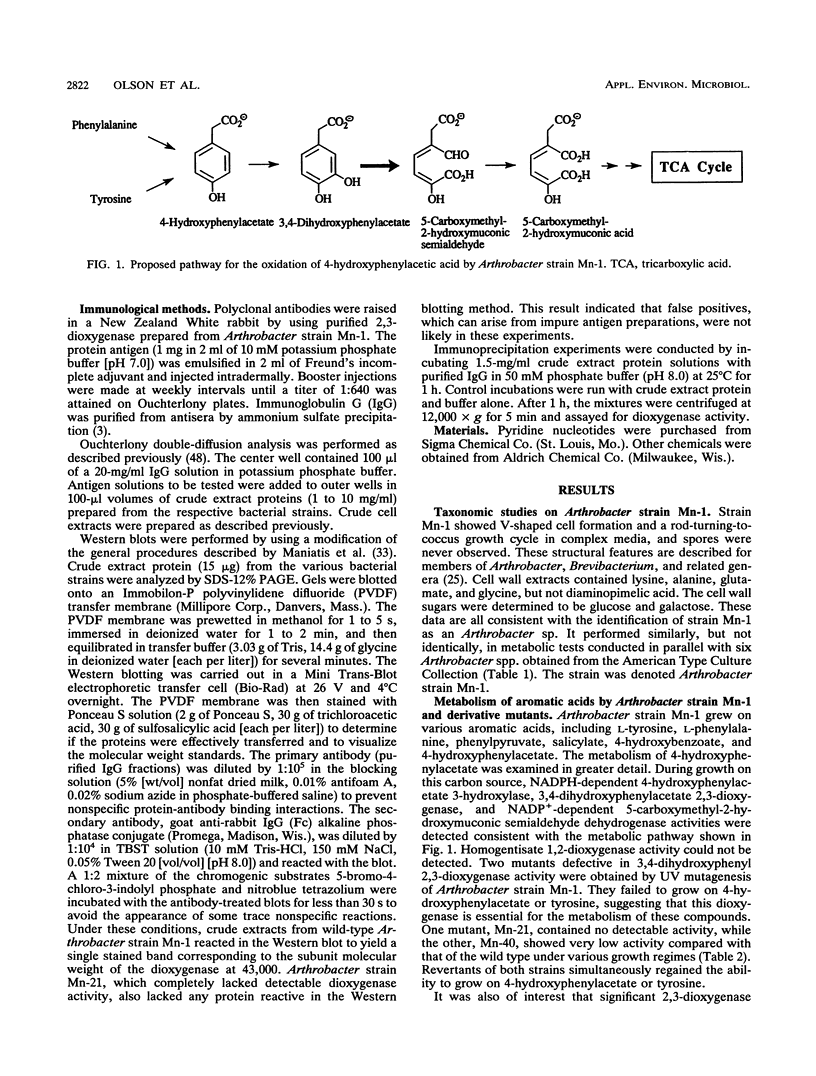
Abstract
Many bacteria biosynthesize 3,4-dihydroxyphenylacetate 2,3-dioxygenases for growth on aromatic acids, but gram-negative organisms have been most extensively studied. A gram-positive strain containing 2,3-dioxygenase activity was identified as Arthrobacter strain Mn-1. The 2,3-dioxygenase from strain Mn-1 was purified to homogeneity by fast protein liquid chromatography with a Mono Q anion-exchange column. Rabbit polyclonal antidioxygenase antibodies were prepared. Ouchterlony double-diffusion and Western blotting (immunoblotting) protocols were used to probe the distribution of the Mn-1 dioxygenase antigen in soil bacteria. Fourteen 2,3-dioxygenase-containing Bacillus and Pseudomonas strains did not contain immunologically cross-reactive proteins. Six of eight Arthrobacter strains contained 2,3-dioxygenase activity, and all of them produced cross-reactive proteins. The data presented here suggest that a unique type of dioxygenase is geographically widespread but is taxonomically confined to Arthrobacter soil bacteria.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADACHI K., TAKEDA Y., SENOH S., KITA H. METABOLISM OF P-HYDROXYPHENYLACETIC ACID IN PSEUDOMONAS OVALIS. Biochim Biophys Acta. 1964 Dec 9;93:483–493. doi: 10.1016/0304-4165(64)90332-0. [DOI] [PubMed] [Google Scholar]
- Arciero D. M., Lipscomb J. D., Huynh B. H., Kent T. A., Münck E. EPR and Mössbauer studies of protocatechuate 4,5-dioxygenase. Characterization of a new Fe2+ environment. J Biol Chem. 1983 Dec 25;258(24):14981–14991. [PubMed] [Google Scholar]
- BECKER B., LECHEVALIER M. P., GORDON R. E., LECHEVALIER H. A. RAPID DIFFERENTIATION BETWEEN NOCARDIA AND STREPTOMYCES BY PAPER CHROMATOGRAPHY OF WHOLE-CELL HYDROLYSATES. Appl Microbiol. 1964 Sep;12:421–423. doi: 10.1128/am.12.5.421-423.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour M. G., Bayly R. C. Control of meta-cleavage degradation of 4-hydroxyphenylacetate in Pseudomonas putida. J Bacteriol. 1981 Sep;147(3):844–850. doi: 10.1128/jb.147.3.844-850.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakley E. R. The catabolism of L-tyrosine by an Arthrobacter sp. Can J Microbiol. 1977 Sep;23(9):1128–1139. doi: 10.1139/m77-169. [DOI] [PubMed] [Google Scholar]
- Boone C. J., Pine L. Rapid method for characterization of actinomycetes by cell wall composition. Appl Microbiol. 1968 Feb;16(2):279–284. doi: 10.1128/am.16.2.279-284.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Conn H. J., Dimmick I. Soil Bacteria Similar in Morphology to Mycobacterium and Corynebacterium. J Bacteriol. 1947 Sep;54(3):291–303. doi: 10.1128/jb.54.3.291-303.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. A., Skinner M. A. Catabolism of 3- and 4-hydroxyphenylacetate by the 3,4-dihydroxyphenylacetate pathway in Escherichia coli. J Bacteriol. 1980 Jul;143(1):302–306. doi: 10.1128/jb.143.1.302-306.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford R. L., Bromley J. W., Perkins-Olson P. E. Catabolism of protocatechuate by Bacillus macerans. Appl Environ Microbiol. 1979 Mar;37(3):614–618. doi: 10.1128/aem.37.3.614-618.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuskey S. M., Olsen R. H. Catabolism of aromatic biogenic amines by Pseudomonas aeruginosa PAO1 via meta cleavage of homoprotocatechuic acid. J Bacteriol. 1988 Jan;170(1):393–399. doi: 10.1128/jb.170.1.393-399.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagley S. A biochemical approach to some problems of environmental pollution. Essays Biochem. 1975;11:81–138. [PubMed] [Google Scholar]
- Durham D. R., Stirling L. A., Ornston L. N., Perry J. J. Intergeneric evolutionary homology revealed by the study of protocatechuate 3,4-dioxygenase from Azotobacter vinelandii. Biochemistry. 1980 Jan 8;19(1):149–155. doi: 10.1021/bi00542a023. [DOI] [PubMed] [Google Scholar]
- ENSIGN J. C., RITTENBERG S. C. A CRYSTALLINE PIGMENT PRODUCED FROM 2-HYDROXYPYRIDINE BY ARTHROBACTER CRYSTALLOPOIETES N.SP. Arch Mikrobiol. 1963 Dec 10;47:137–153. doi: 10.1007/BF00422519. [DOI] [PubMed] [Google Scholar]
- Harayama S., Rekik M. Bacterial aromatic ring-cleavage enzymes are classified into two different gene families. J Biol Chem. 1989 Sep 15;264(26):15328–15333. [PubMed] [Google Scholar]
- Jamaluddin M. P. Purification and properties of homoprotocatechuate 2,3-dioxygenase from Bacillus stearothermophilus. J Bacteriol. 1977 Feb;129(2):690–697. doi: 10.1128/jb.129.2.690-697.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H. Crystallization and some properties of 3,4-dihydroxyphenylacetate 2,3-oxygenase from Pseudomonas ovalis. J Biochem. 1965 Aug;58(2):116–122. doi: 10.1093/oxfordjournals.jbchem.a128172. [DOI] [PubMed] [Google Scholar]
- Kolenbrander P. E., Weinberger M. 2-Hydroxypyridine metabolism and pigment formation in three Arthrobacter species. J Bacteriol. 1977 Oct;132(1):51–59. doi: 10.1128/jb.132.1.51-59.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutty R. K., Devi N. A., Veeraswamy M., Ramesh S., Rao P. V. Degradation of (+/-)-synephrine by Arthrobacter synephrinum. Oxidation of 3,4-dihydroxyphenylacetate to 2-hydroxy-5-carboxymethyl-muconate semialdehyde. Biochem J. 1977 Oct 1;167(1):163–170. doi: 10.1042/bj1670163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee Y. L., Dagley S. Comparison of two dioxygenases from Pseudomonas putida. J Bacteriol. 1977 Sep;131(3):1016–1017. doi: 10.1128/jb.131.3.1016-1017.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks T. S., Smith A. R., Quirk A. V. Degradation of 4-Chlorobenzoic Acid by Arthrobacter sp. Appl Environ Microbiol. 1984 Nov;48(5):1020–1025. doi: 10.1128/aem.48.5.1020-1025.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidle E. L., Hartnett C., Bonitz S., Ornston L. N. DNA sequence of the Acinetobacter calcoaceticus catechol 1,2-dioxygenase I structural gene catA: evidence for evolutionary divergence of intradiol dioxygenases by acquisition of DNA sequence repetitions. J Bacteriol. 1988 Oct;170(10):4874–4880. doi: 10.1128/jb.170.10.4874-4880.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono-Kamimoto M. Studies on 3,4-dihydroxyphenylacetate 2,3-dioxygenase. I. Role of iron, substrate binding, and some other properties. J Biochem. 1973 Nov;74(5):1049–1059. [PubMed] [Google Scholar]
- Que L., Jr, Widom J., Crawford R. L. 3,4-Dihydroxyphenylacetate 2,3-dioxygenase. A manganese(II) dioxygenase from Bacillus brevis. J Biol Chem. 1981 Nov 10;256(21):10941–10944. [PubMed] [Google Scholar]
- Roper D. I., Cooper R. A. Subcloning and nucleotide sequence of the 3,4-dihydroxyphenylacetate (homoprotocatechuate) 2,3-dioxygenase gene from Escherichia coli C. FEBS Lett. 1990 Nov 26;275(1-2):53–57. doi: 10.1016/0014-5793(90)81437-s. [DOI] [PubMed] [Google Scholar]
- SGUROS P. L. Microbial transformations of the tobacco alkaloids. I. Cultural and morphological characteristics of a nicotinophile. J Bacteriol. 1955 Jan;69(1):28–37. doi: 10.1128/jb.69.1.28-37.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparnins V. L., Chapman P. J. Catabolism of L-tyrosine by the homoprotocatechuate pathway in gram-positive bacteria. J Bacteriol. 1976 Jul;127(1):362–366. doi: 10.1128/jb.127.1.362-366.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparnins V. L., Chapman P. J., Dagley S. Bacterial degradation of 4-hydroxyphenylacetic acid and homoprotocatechuic acid. J Bacteriol. 1974 Oct;120(1):159–167. doi: 10.1128/jb.120.1.159-167.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker J. W., Lipscomb J. D., Kent T. A., Münck E. Brevibacterium fuscum protocatechuate 3,4-dioxygenase. Purification, crystallization, and characterization. J Biol Chem. 1984 Apr 10;259(7):4466–4475. [PubMed] [Google Scholar]