Abstract
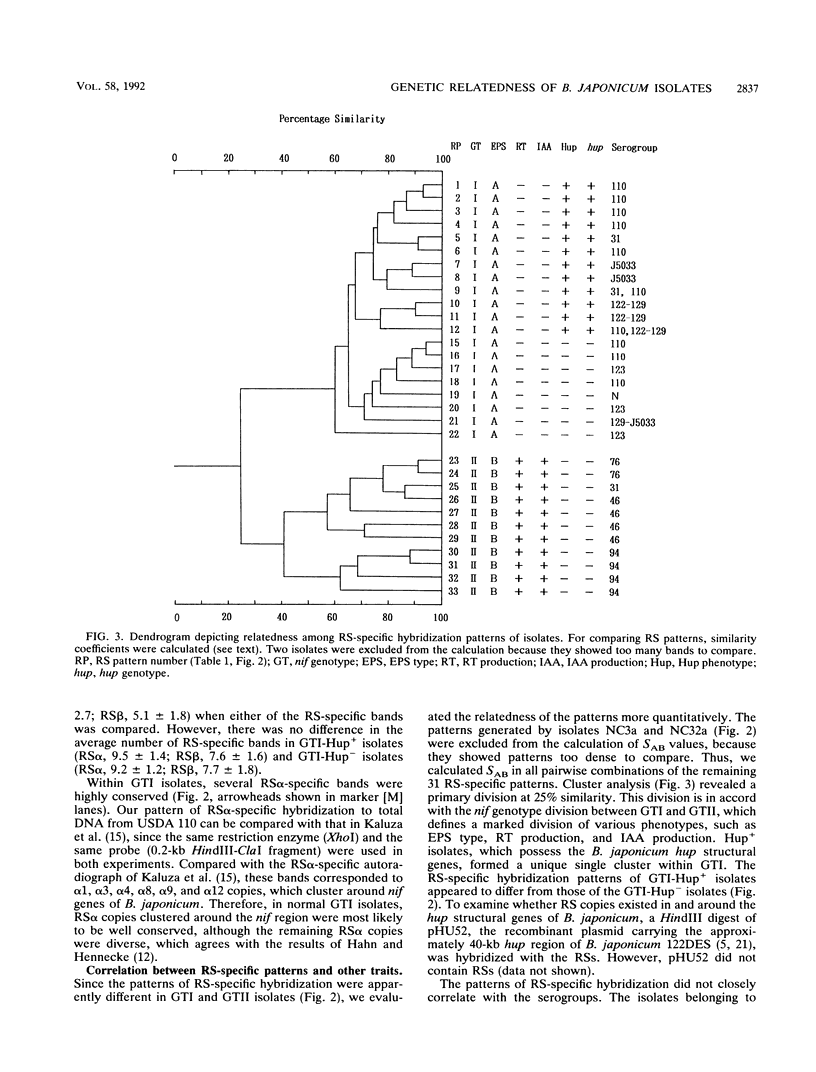
Forty-nine isolates of Bradyrhizobium japonicum indigenous to a field where soybeans were grown for 45 years without inoculation were characterized by using four DNA hybridization probes from B. japonicum. nifDK-specific hybridization clearly divided the isolates into two divergent groups. Diversity in repeated-sequence (RS)-specific hybridization was observed; 44 isolates derived from 41 nodules were divided into 33 different RS fingerprint groups. Cluster analysis showed that the RS fingerprints were correlated with the nif and hup genotypes. We found multiple bands of RS-specific hybridization for two isolates that differed from the patterns of the other isolates. These results suggest that RS fingerprinting is a valuable tool for evaluating the genetic structure of indigenous B. japonicum populations.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beringer J. E. R factor transfer in Rhizobium leguminosarum. J Gen Microbiol. 1974 Sep;84(1):188–198. doi: 10.1099/00221287-84-1-188. [DOI] [PubMed] [Google Scholar]
- Chen C. W., Thomas C. A., Jr Recovery of DNA segments from agarose gels. Anal Biochem. 1980 Jan 15;101(2):339–341. doi: 10.1016/0003-2697(80)90197-9. [DOI] [PubMed] [Google Scholar]
- Evans H. J., Harker A. R., Papen H., Russell S. A., Hanus F. J., Zuber M. Physiology, biochemistry, and genetics of the uptake hydrogenase in rhizobia. Annu Rev Microbiol. 1987;41:335–361. doi: 10.1146/annurev.mi.41.100187.002003. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fuhrmann J. Symbiotic effectiveness of indigenous soybean bradyrhizobia as related to serological, morphological, rhizobitoxine, and hydrogenase phenotypes. Appl Environ Microbiol. 1990 Jan;56(1):224–229. doi: 10.1128/aem.56.1.224-229.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. A., Weber R. P. COLORIMETRIC ESTIMATION OF INDOLEACETIC ACID. Plant Physiol. 1951 Jan;26(1):192–195. doi: 10.1104/pp.26.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M., Hennecke H. Conservation of a symbiotic DNA region in soybean root nodule bacteria. Appl Environ Microbiol. 1987 Sep;53(9):2253–2255. doi: 10.1128/aem.53.9.2253-2255.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M., Hennecke H. Mapping of a Bradyrhizobium japonicum DNA Region Carrying Genes for Symbiosis and an Asymmetric Accumulation of Reiterated Sequences. Appl Environ Microbiol. 1987 Sep;53(9):2247–2252. doi: 10.1128/aem.53.9.2247-2252.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaluza K., Hahn M., Hennecke H. Repeated sequences similar to insertion elements clustered around the nif region of the Rhizobium japonicum genome. J Bacteriol. 1985 May;162(2):535–542. doi: 10.1128/jb.162.2.535-542.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser H. H., Weber D. F., Uratsu S. L. Rhizobium japonicum Serogroup and Hydrogenase Phenotype Distribution in 12 States. Appl Environ Microbiol. 1984 Apr;47(4):613–615. doi: 10.1128/aem.47.4.613-615.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert G. R., Cantrell M. A., Hanus F. J., Russell S. A., Haddad K. R., Evans H. J. Intra- and interspecies transfer and expression of Rhizobium japonicum hydrogen uptake genes and autotrophic growth capability. Proc Natl Acad Sci U S A. 1985 May;82(10):3232–3236. doi: 10.1073/pnas.82.10.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel K. D., Brill W. J. Diversity and Dynamics of Indigenous Rhizobium japonicum Populations. Appl Environ Microbiol. 1980 Nov;40(5):931–938. doi: 10.1128/aem.40.5.931-938.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens L. D., Wright D. A. Production of the Soybean-Chlorosis Toxin by Rhizobium japonicum in Pure Culture. Plant Physiol. 1965 Sep;40(5):931–933. doi: 10.1104/pp.40.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J., Brown G. G., Verma D. P. Slow-growing Rhizobium japonicum comprises two highly divergent symbiotic types. J Bacteriol. 1985 Jul;163(1):148–154. doi: 10.1128/jb.163.1.148-154.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]