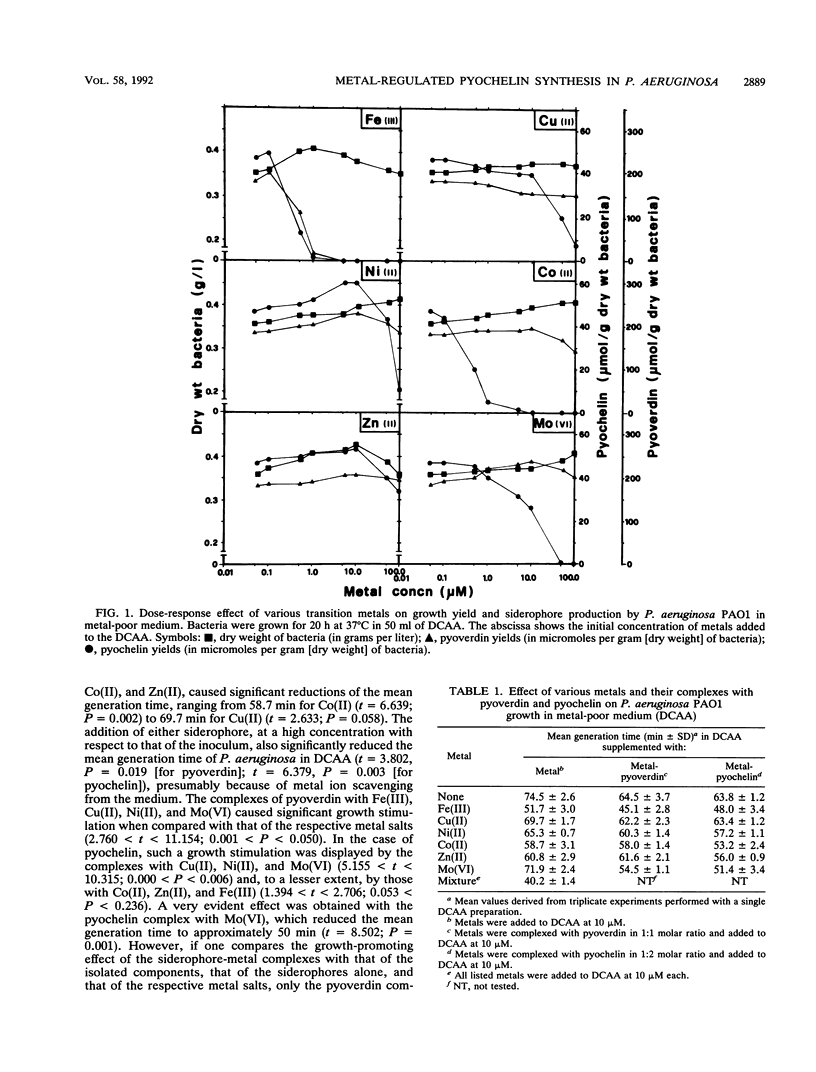
Abstract
Pseudomonas aeruginosa synthesizes two siderophores, pyochelin and pyoverdin, characterized by widely different structures, physicochemical properties, and affinities for Fe(III). Titration experiments showed that pyochelin, which is endowed with a relatively low affinity for Fe(III), binds other transition metals, such as Cu(II), Co(II), Mo(VI), and Ni(II), with appreciable affinity. In line with these observations, Fe(III) and Co(II) at 10 microM or Mo(VI), Ni(II), and Cu(II) at 100 microM repressed pyochelin synthesis and reduced expression of iron-regulated outer membrane proteins of 75, 68, and 14 kDa. In contrast, pyoverdin synthesis and expression of the 80-kDa receptor protein were affected only by Fe(III). All of the metals tested, except Mo(VI), significantly promoted P. aeruginosa growth in metal-poor medium; Mo(VI), Ni(II), and Co(II) were more efficient as pyochelin complexes than the free metal ions and the siderophore. The observed correlation between the affinity of pyochelin for Fe(III), Co(II), and Mo(VI) and the functional effects of these metals indicates that pyochelin may play a role in their delivery to P. aeruginosa.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ankenbauer R. G., Cox C. D. Isolation and characterization of Pseudomonas aeruginosa mutants requiring salicylic acid for pyochelin biosynthesis. J Bacteriol. 1988 Nov;170(11):5364–5367. doi: 10.1128/jb.170.11.5364-5367.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankenbauer R. G., Staley A. L., Rinehart K. L., Cox C. D. Mutasynthesis of siderophore analogues by Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1878–1882. doi: 10.1073/pnas.88.5.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskot G., Taraz K., Budzikiewicz H. Siderophore vom Pyoverdin-Typ aus Pseudomonas aeruginosa. Z Naturforsch C. 1986 May-Jun;41(5-6):497–506. [PubMed] [Google Scholar]
- Cox C. D., Adams P. Siderophore activity of pyoverdin for Pseudomonas aeruginosa. Infect Immun. 1985 Apr;48(1):130–138. doi: 10.1128/iai.48.1.130-138.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D., Graham R. Isolation of an iron-binding compound from Pseudomonas aeruginosa. J Bacteriol. 1979 Jan;137(1):357–364. doi: 10.1128/jb.137.1.357-364.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan P., Hercberg S., Touitou Y. The activity of tissue enzymes in iron-deficient rat and man: an overview. Comp Biochem Physiol B. 1984;77(4):647–653. doi: 10.1016/0305-0491(84)90292-x. [DOI] [PubMed] [Google Scholar]
- Guterman S. K. Colicin B: mode of action and inhibition by enterochelin. J Bacteriol. 1973 Jun;114(3):1217–1224. doi: 10.1128/jb.114.3.1217-1224.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs D. E., Young L., Poole K. Pyochelin-mediated iron transport in Pseudomonas aeruginosa: involvement of a high-molecular-mass outer membrane protein. Infect Immun. 1991 Oct;59(10):3680–3684. doi: 10.1128/iai.59.10.3680-3684.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kustin K., Liu S. T. Kinetics and complex formation of molybdate with catechol. J Am Chem Soc. 1973 Apr 18;95(8):2487–2491. doi: 10.1021/ja00789a012. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Meyer J. M., Hohnadel D., Khan A., Cornelis P. Pyoverdin-facilitated iron uptake in Pseudomonas aeruginosa: immunological characterization of the ferripyoverdin receptor. Mol Microbiol. 1990 Aug;4(8):1401–1405. doi: 10.1111/j.1365-2958.1990.tb00719.x. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Poole K., Neshat S., Heinrichs D. Pyoverdine-mediated iron transport in Pseudomonas aeruginosa: involvement of a high-molecular-mass outer membrane protein. FEMS Microbiol Lett. 1991 Feb;62(1):1–5. [PubMed] [Google Scholar]
- Sokol P. A., Woods D. E. Demonstration of an iron-siderophore-binding protein in the outer membrane of Pseudomonas aeruginosa. Infect Immun. 1983 May;40(2):665–669. doi: 10.1128/iai.40.2.665-669.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. H. Novel iron uptake system specified by ColV plasmids: an important component in the virulence of invasive strains of Escherichia coli. Infect Immun. 1979 Dec;26(3):925–932. doi: 10.1128/iai.26.3.925-932.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



