Abstract
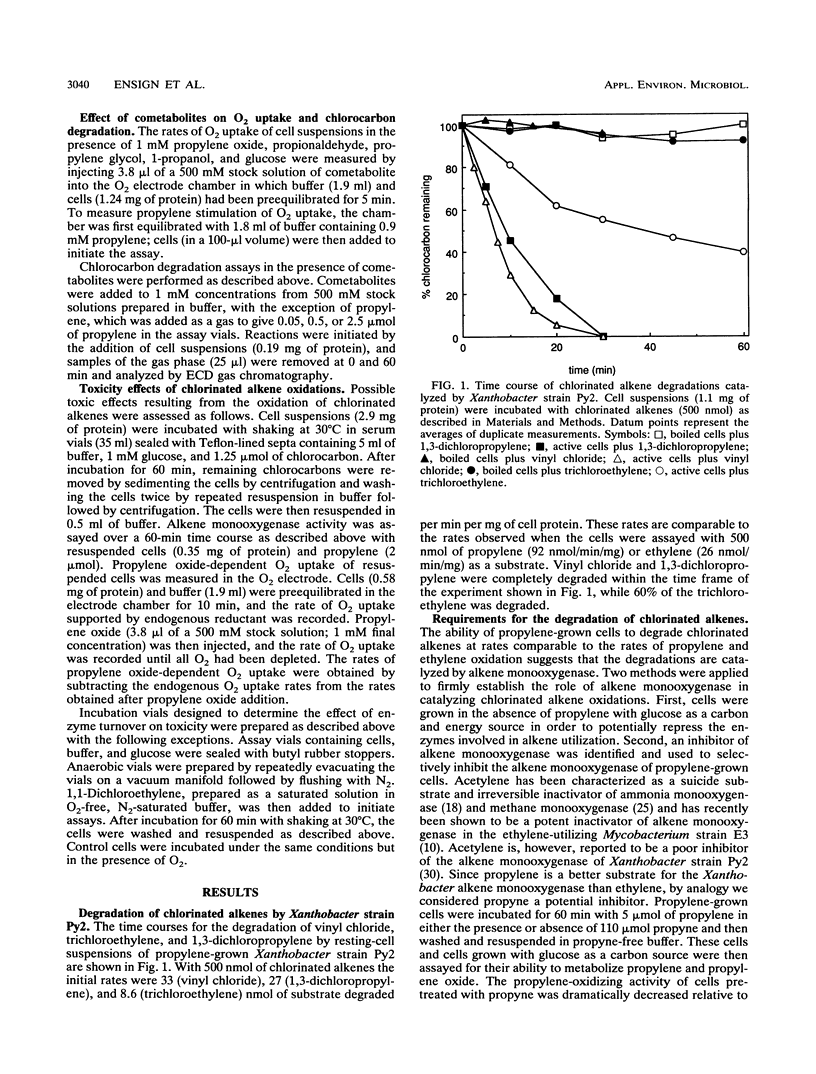
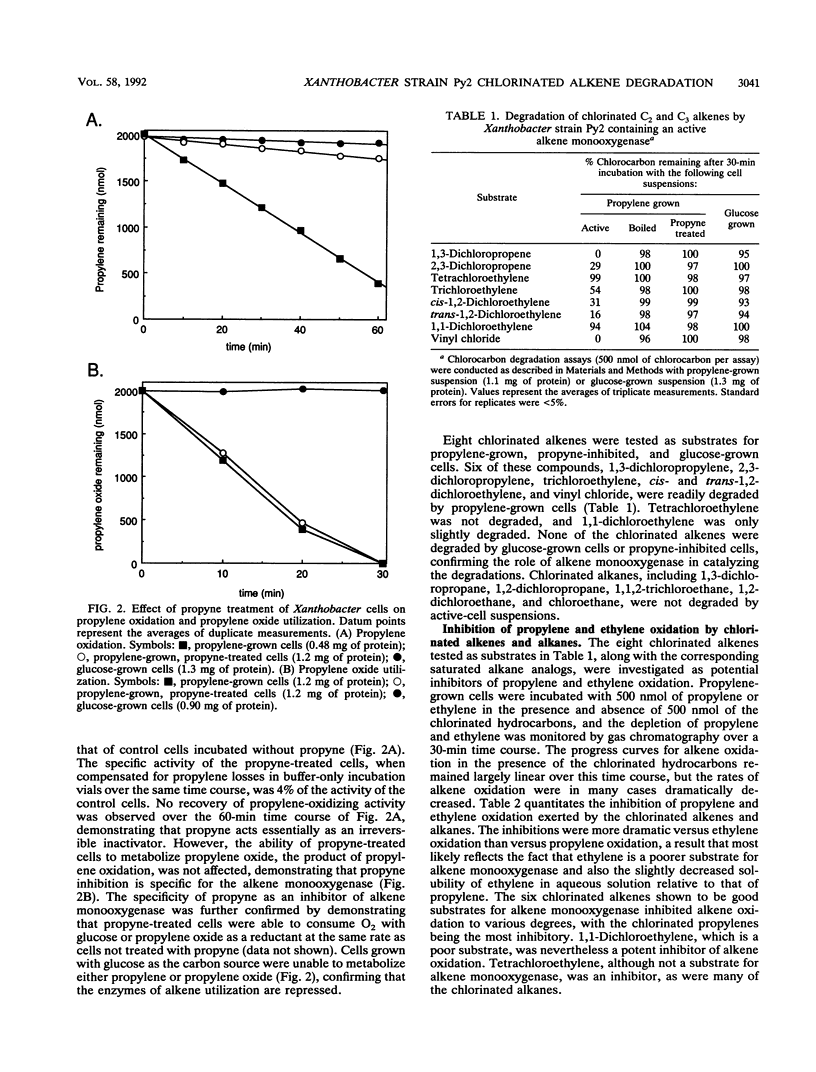
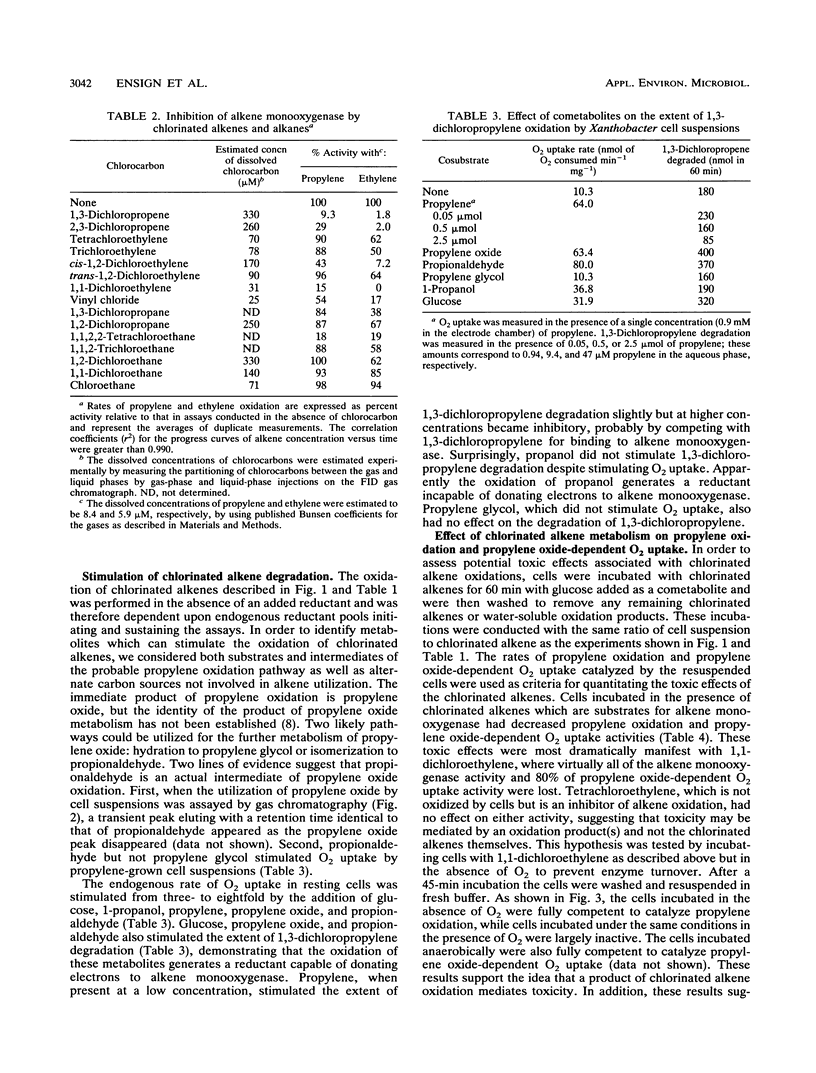
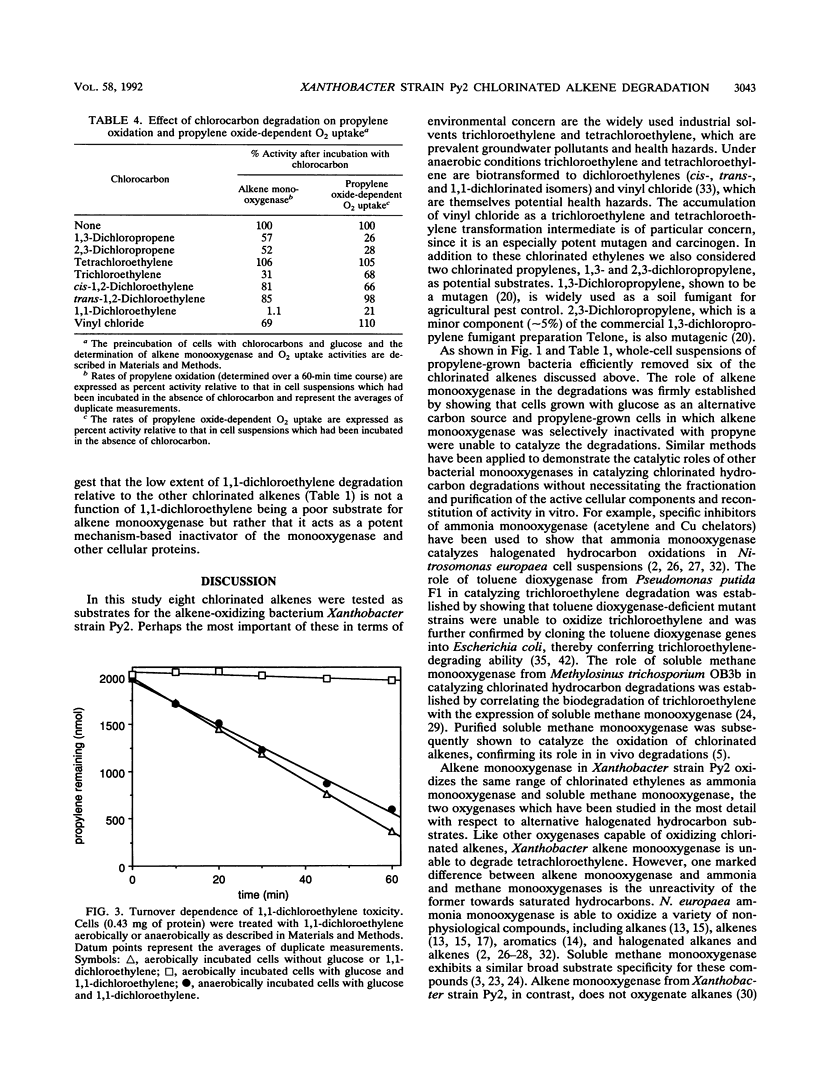
Propylene-grown Xanthobacter cells (strain Py2) degraded several chlorinated alkenes of environmental concern, including trichloroethylene, 1-chloroethylene (vinyl chloride), cis- and trans-1,2-dichloroethylene, 1,3-dichloropropylene, and 2,3-dichloropropylene. 1,1-Dichloroethylene was not degraded efficiently, while tetrachloroethylene was not degraded. The role of alkene monooxygenase in catalyzing chlorinated alkene degradations was established by demonstrating that glucose-grown cells which lack alkene monooxygenase and propylene-grown cells in which alkene monooxygenase was selectively inactivated by propyne were unable to degrade the compounds. C2 and C3 chlorinated alkanes were not oxidized by alkene monooxygenase, but a number of these compounds were inhibitors of propylene and ethylene oxidation, suggesting that they compete for binding to the enzyme. A number of metabolites enhanced the rate of degradation of chlorinated alkenes, including propylene oxide, propionaldehyde, and glucose. Propylene stimulated chlorinated alkene oxidation slightly when present at a low concentration but became inhibitory at higher concentrations. Toxic effects associated with chlorinated alkene oxidations were determined by measuring the propylene oxidation and propylene oxide-dependent O2 uptake rates of cells previously incubated with chlorinated alkenes. Compounds which were substrates for alkene monooxygenase exhibited various levels of toxicity, with 1,1-dichloroethylene and trichloroethylene being the most potent inactivators of propylene oxidation and 1,3- and 2,3-dichloropropylene being the most potent inactivators of propylene oxide-dependent O2 uptake. No toxic effects were seen when cells were incubated with chlorinated alkenes anaerobically, indicating that the product(s) of chlorinated alkene oxidation mediates toxicity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez-Cohen L., McCarty P. L. Effects of toxicity, aeration, and reductant supply on trichloroethylene transformation by a mixed methanotrophic culture. Appl Environ Microbiol. 1991 Jan;57(1):228–235. doi: 10.1128/aem.57.1.228-235.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arciero D., Vannelli T., Logan M., Hooper A. B. Degradation of trichloroethylene by the ammonia-oxidizing bacterium Nitrosomonas europaea. Biochem Biophys Res Commun. 1989 Mar 15;159(2):640–643. doi: 10.1016/0006-291x(89)90042-9. [DOI] [PubMed] [Google Scholar]
- Colby J., Stirling D. I., Dalton H. The soluble methane mono-oxygenase of Methylococcus capsulatus (Bath). Its ability to oxygenate n-alkanes, n-alkenes, ethers, and alicyclic, aromatic and heterocyclic compounds. Biochem J. 1977 Aug 1;165(2):395–402. doi: 10.1042/bj1650395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewers J., Freier-Schröder D., Knackmuss H. J. Selection of trichloroethene (TCE) degrading bacteria that resist inactivation by TCE. Arch Microbiol. 1990;154(4):410–413. doi: 10.1007/BF00276540. [DOI] [PubMed] [Google Scholar]
- Fox B. G., Borneman J. G., Wackett L. P., Lipscomb J. D. Haloalkene oxidation by the soluble methane monooxygenase from Methylosinus trichosporium OB3b: mechanistic and environmental implications. Biochemistry. 1990 Jul 10;29(27):6419–6427. doi: 10.1021/bi00479a013. [DOI] [PubMed] [Google Scholar]
- Hartmans S., Weber F. J., Somhorst D. P., de Bont J. A. Alkene monooxygenase from Mycobacterium: a multicomponent enzyme. J Gen Microbiol. 1991 Nov;137(11):2555–2560. doi: 10.1099/00221287-137-11-2555. [DOI] [PubMed] [Google Scholar]
- Hartmans S., de Bont J. A., Harder W. Microbial metabolism of short-chain unsaturated hydrocarbons. FEMS Microbiol Rev. 1989 Sep;5(3):235–264. doi: 10.1016/0168-6445(89)90034-x. [DOI] [PubMed] [Google Scholar]
- Henschler D., Hoos W. R., Fetz H., Dallmeier E., Metzler M. Reactions of trichloroethylene epoxide in aqueous systems. Biochem Pharmacol. 1979;28(4):543–548. doi: 10.1016/0006-2952(79)90251-x. [DOI] [PubMed] [Google Scholar]
- Hyman M. R., Murton I. B., Arp D. J. Interaction of Ammonia Monooxygenase from Nitrosomonas europaea with Alkanes, Alkenes, and Alkynes. Appl Environ Microbiol. 1988 Dec;54(12):3187–3190. doi: 10.1128/aem.54.12.3187-3190.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman M. R., Wood P. M. Methane oxidation by Nitrosomonas europaea. Biochem J. 1983 Apr 15;212(1):31–37. doi: 10.1042/bj2120031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman M. R., Wood P. M. Suicidal inactivation and labelling of ammonia mono-oxygenase by acetylene. Biochem J. 1985 May 1;227(3):719–725. doi: 10.1042/bj2270719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little C. D., Palumbo A. V., Herbes S. E., Lidstrom M. E., Tyndall R. L., Gilmer P. J. Trichloroethylene biodegradation by a methane-oxidizing bacterium. Appl Environ Microbiol. 1988 Apr;54(4):951–956. doi: 10.1128/aem.54.4.951-956.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. E., Guengerich F. P. Oxidation of trichloroethylene by liver microsomal cytochrome P-450: evidence for chlorine migration in a transition state not involving trichloroethylene oxide. Biochemistry. 1982 Mar 2;21(5):1090–1097. doi: 10.1021/bi00534a041. [DOI] [PubMed] [Google Scholar]
- Nelson M. J., Montgomery S. O., Mahaffey W. R., Pritchard P. H. Biodegradation of trichloroethylene and involvement of an aromatic biodegradative pathway. Appl Environ Microbiol. 1987 May;53(5):949–954. doi: 10.1128/aem.53.5.949-954.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenhuis R., Oedzes J. Y., van der Waarde J. J., Janssen D. B. Kinetics of chlorinated hydrocarbon degradation by Methylosinus trichosporium OB3b and toxicity of trichloroethylene. Appl Environ Microbiol. 1991 Jan;57(1):7–14. doi: 10.1128/aem.57.1.7-14.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenhuis R., Vink R. L., Janssen D. B., Witholt B. Degradation of chlorinated aliphatic hydrocarbons by Methylosinus trichosporium OB3b expressing soluble methane monooxygenase. Appl Environ Microbiol. 1989 Nov;55(11):2819–2826. doi: 10.1128/aem.55.11.2819-2826.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasche M. E., Hicks R. E., Hyman M. R., Arp D. J. Oxidation of monohalogenated ethanes and n-chlorinated alkanes by whole cells of Nitrosomonas europaea. J Bacteriol. 1990 Sep;172(9):5368–5373. doi: 10.1128/jb.172.9.5368-5373.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasche M. E., Hyman M. R., Arp D. J. Factors Limiting Aliphatic Chlorocarbon Degradation by Nitrosomonas europaea: Cometabolic Inactivation of Ammonia Monooxygenase and Substrate Specificity. Appl Environ Microbiol. 1991 Oct;57(10):2986–2994. doi: 10.1128/aem.57.10.2986-2994.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasche Madeline E., Hyman Michael R., Arp Daniel J. Biodegradation of Halogenated Hydrocarbon Fumigants by Nitrifying Bacteria. Appl Environ Microbiol. 1990 Aug;56(8):2568–2571. doi: 10.1128/aem.56.8.2568-2571.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien H. C., Brusseau G. A., Hanson R. S., Waclett L. P. Biodegradation of trichloroethylene by Methylosinus trichosporium OB3b. Appl Environ Microbiol. 1989 Dec;55(12):3155–3161. doi: 10.1128/aem.55.12.3155-3161.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannelli T., Logan M., Arciero D. M., Hooper A. B. Degradation of halogenated aliphatic compounds by the ammonia- oxidizing bacterium Nitrosomonas europaea. Appl Environ Microbiol. 1990 Apr;56(4):1169–1171. doi: 10.1128/aem.56.4.1169-1171.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel T. M., McCarty P. L. Biotransformation of tetrachloroethylene to trichloroethylene, dichloroethylene, vinyl chloride, and carbon dioxide under methanogenic conditions. Appl Environ Microbiol. 1985 May;49(5):1080–1083. doi: 10.1128/aem.49.5.1080-1083.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackett L. P., Brusseau G. A., Householder S. R., Hanson R. S. Survey of microbial oxygenases: trichloroethylene degradation by propane-oxidizing bacteria. Appl Environ Microbiol. 1989 Nov;55(11):2960–2964. doi: 10.1128/aem.55.11.2960-2964.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackett L. P., Gibson D. T. Degradation of trichloroethylene by toluene dioxygenase in whole-cell studies with Pseudomonas putida F1. Appl Environ Microbiol. 1988 Jul;54(7):1703–1708. doi: 10.1128/aem.54.7.1703-1708.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackett L. P., Householder S. R. Toxicity of Trichloroethylene to Pseudomonas putida F1 Is Mediated by Toluene Dioxygenase. Appl Environ Microbiol. 1989 Oct;55(10):2723–2725. doi: 10.1128/aem.55.10.2723-2725.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


