Abstract
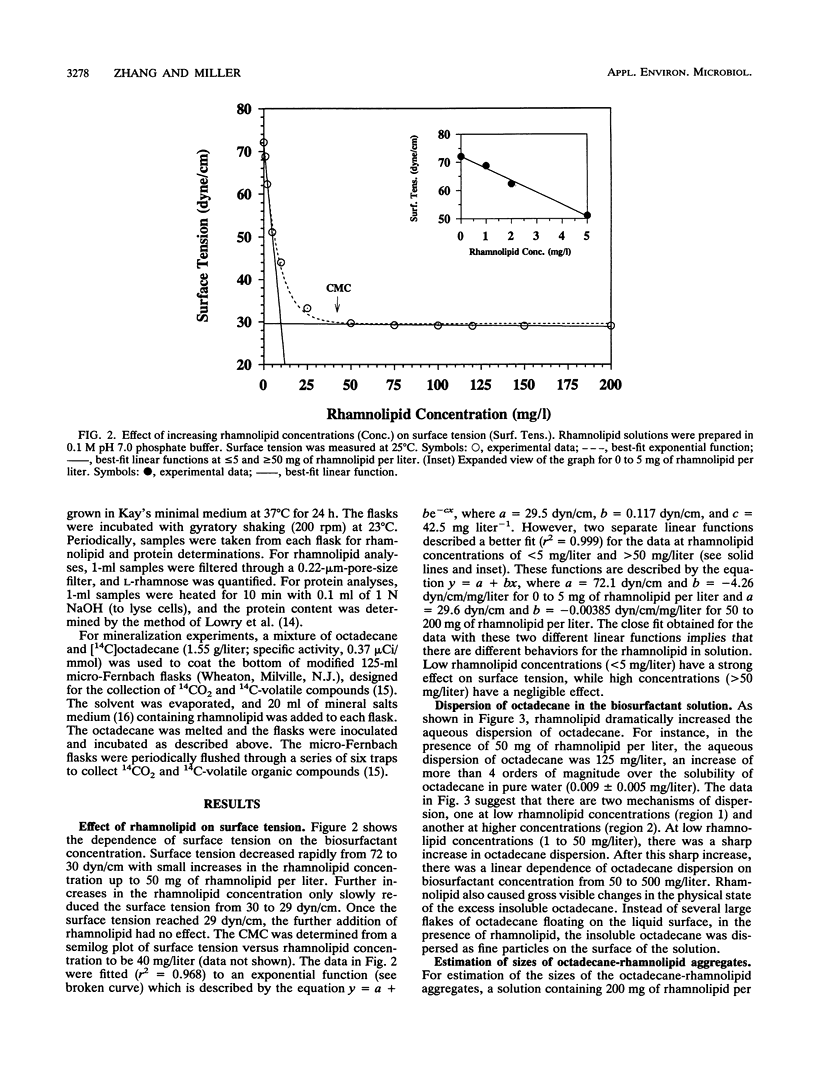
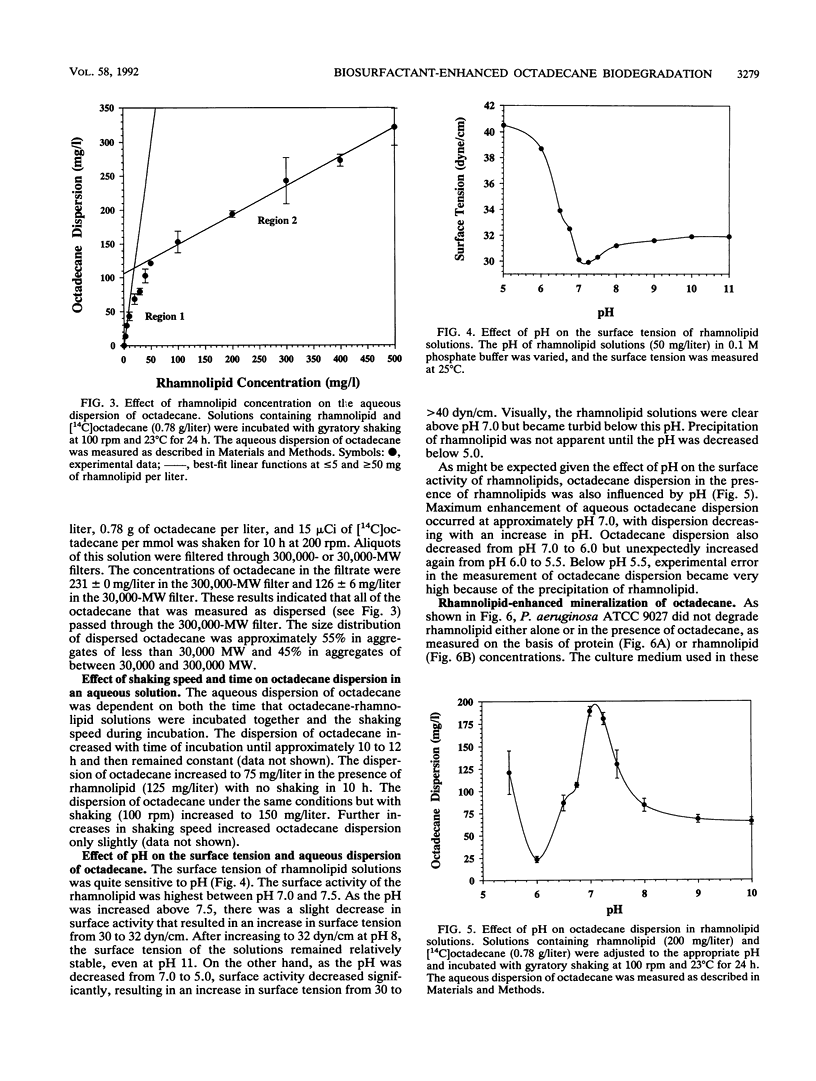
A microbial surfactant (biosurfactant) was investigated for its potential to enhance bioavailability and, hence, the biodegradation of octadecane. The rhamnolipid biosurfactant used in this study was extracted from culture supernatants after growth of Pseudomonas aeruginosa ATCC 9027 in phosphate-limited proteose peptone-glucose-ammonium salts medium. Dispersion of octadecane in aqueous solutions was dramatically enhanced by 300 mg of the rhamnolipid biosurfactant per liter, increasing by a factor of more than 4 orders of magnitude, from 0.009 to > 250 mg/liter. The relative enhancement of octadecane dispersion was much greater at low rhamnolipid concentrations than at high concentrations. Rhamnolipid-enhanced octadecane dispersion was found to be dependent on pH and shaking speed. Biodegradation experiments done with an initial octadecane concentration of 1,500 mg/liter showed that 20% of the octadecane was mineralized in 84 h in the presence of 300 mg of rhamnolipid per liter, compared with only 5% octadecane mineralization when no surfactant was present. These results indicate that rhamnolipids may have potential for facilitating the bioremediation of sites contaminated with hydrocarbons having limited water solubility.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheng K. J., Ingram J. M., Costerton J. W. Release of alkaline phosphatase from cells of Pseudomonas aeruginosa by manipulation of cation concentration and of pH. J Bacteriol. 1970 Nov;104(2):748–753. doi: 10.1128/jb.104.2.748-753.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. R., Hayashi J. A. Structure of a rhamnolipid from Pseudomonas aeruginosa. Arch Biochem Biophys. 1965 Aug;111(2):415–421. doi: 10.1016/0003-9861(65)90204-3. [DOI] [PubMed] [Google Scholar]
- Guerra-Santos L., Käppeli O., Fiechter A. Pseudomonas aeruginosa biosurfactant production in continuous culture with glucose as carbon source. Appl Environ Microbiol. 1984 Aug;48(2):301–305. doi: 10.1128/aem.48.2.301-305.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Honda H., Tomita F., Suzuki T. Rhamnolipids produced by Pseudomonas aeruginosa grown on n-paraffin (mixture of C 12 , C 13 and C 14 fractions). J Antibiot (Tokyo) 1971 Dec;24(12):855–859. doi: 10.7164/antibiotics.24.855. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marinucci A. C., Bartha R. Apparatus for monitoring the mineralization of volatile C-labeled compounds. Appl Environ Microbiol. 1979 Nov;38(5):1020–1022. doi: 10.1128/aem.38.5.1020-1022.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. M., Bartha R. Evidence from liposome encapsulation for transport-limited microbial metabolism of solid alkanes. Appl Environ Microbiol. 1989 Feb;55(2):269–274. doi: 10.1128/aem.55.2.269-274.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendell N. B., Taylor G. W., Somerville M., Todd H., Wilson R., Cole P. J. Characterisation of Pseudomonas rhamnolipids. Biochim Biophys Acta. 1990 Jul 16;1045(2):189–193. doi: 10.1016/0005-2760(90)90150-v. [DOI] [PubMed] [Google Scholar]
- WARREN R. A., ELLS A. F., CAMPBELL J. J. Endogenous respiration of Pseudomonas aeruginosa. J Bacteriol. 1960 Jun;79:875–879. doi: 10.1128/jb.79.6.875-879.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]


