Abstract
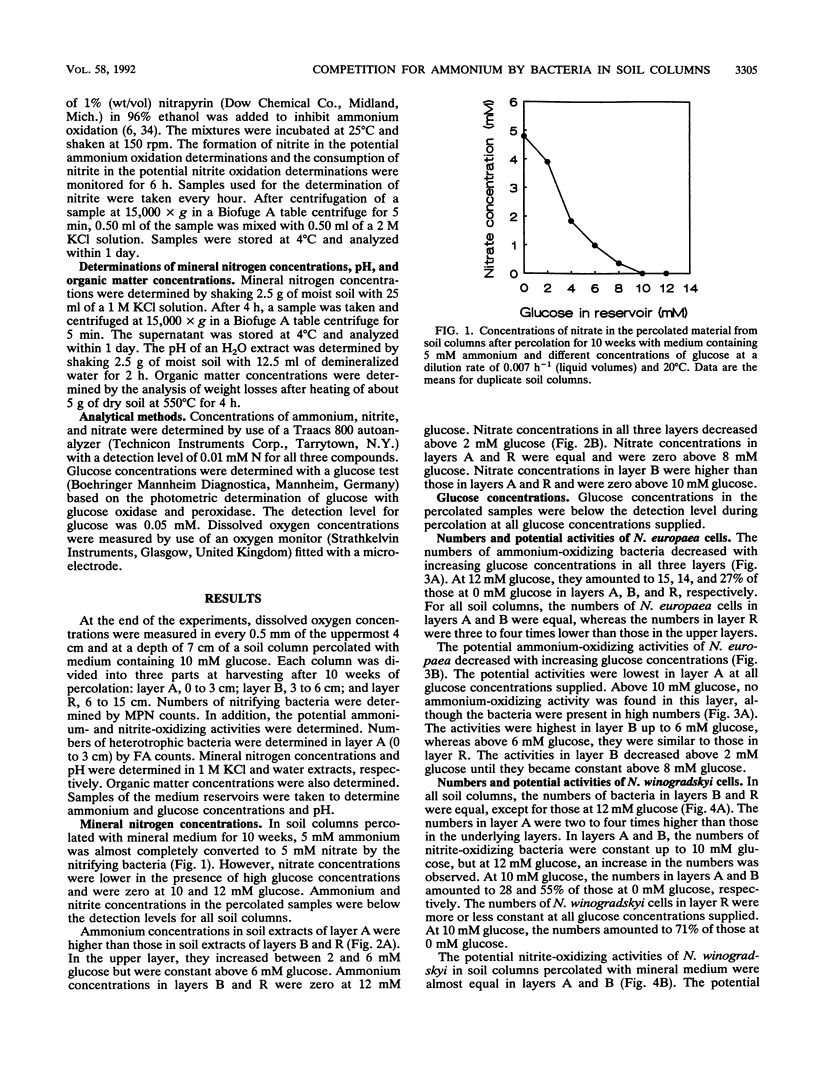
Although the absence of nitrate formation in grassland soils rich in organic matter has often been reported, low numbers of nitrifying bacteria are still found in these soils. To obtain more insight into these observations, we studied the competition for limiting amounts of ammonium between the chemolithotrophic ammonium-oxidizing species Nitrosomonas europaea and the heterotrophic species Arthrobacter globiformis in the presence of Nitrobacter winogradskyi with soil columns containing calcareous sandy soil. The soil columns were percolated continuously at a dilution rate of 0.007 h-1, based on liquid volumes, with medium containing 5 mM ammonium and different amounts of glucose ranging from 0 to 12 mM.A. globiformis was the most competitive organism for limiting amounts of ammonium. The numbers of N. europaea and N. winogradskyi cells were lower at higher glucose concentrations, and the potential ammonium-oxidizing activities in the uppermost 3 cm of the soil columns were nonexistent when at least 10 mM glucose was present in the reservoir, although 107 nitrifying cells per g of dry soil were still present. This result demonstrated that there was no correlation between the numbers of nitrifying bacteria and their activities. The numbers and activities of N. winogradskyi cells decreased less than those of N. europaea cells in all layers of the soil columns, probably because of heterotrophic growth of the nitrite-oxidizing bacteria on organic substrates excreted by the heterotrophic bacteria or because of nitrate reduction at reduced oxygen concentrations by the nitrite-oxidizing bacteria. Our conclusion was that the nitrifying bacteria were less competitive than the heterotrophic bacteria for ammonium in soil columns but that they survived as viable inactive cells. Inactive nitrifying bacteria may also be found in the rhizosphere of grassland plants, which is rich in organic carbon. They are possibly reactivated during periods of net mineralization.
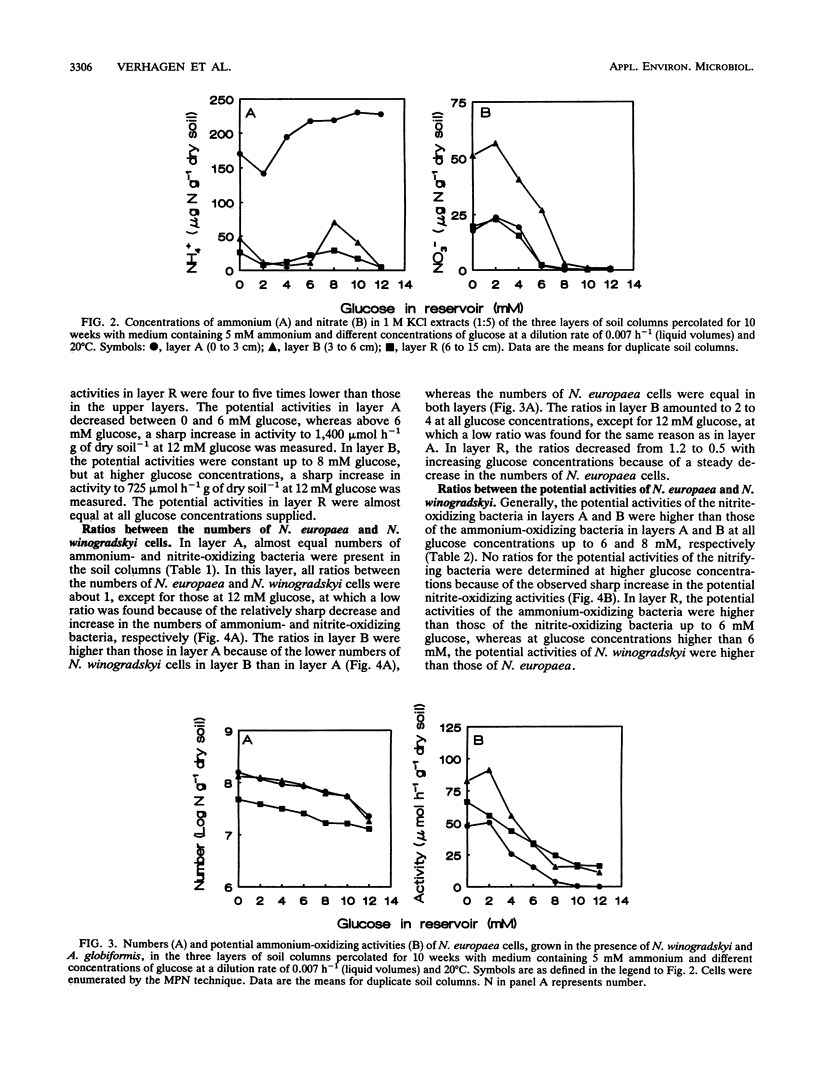
Full text
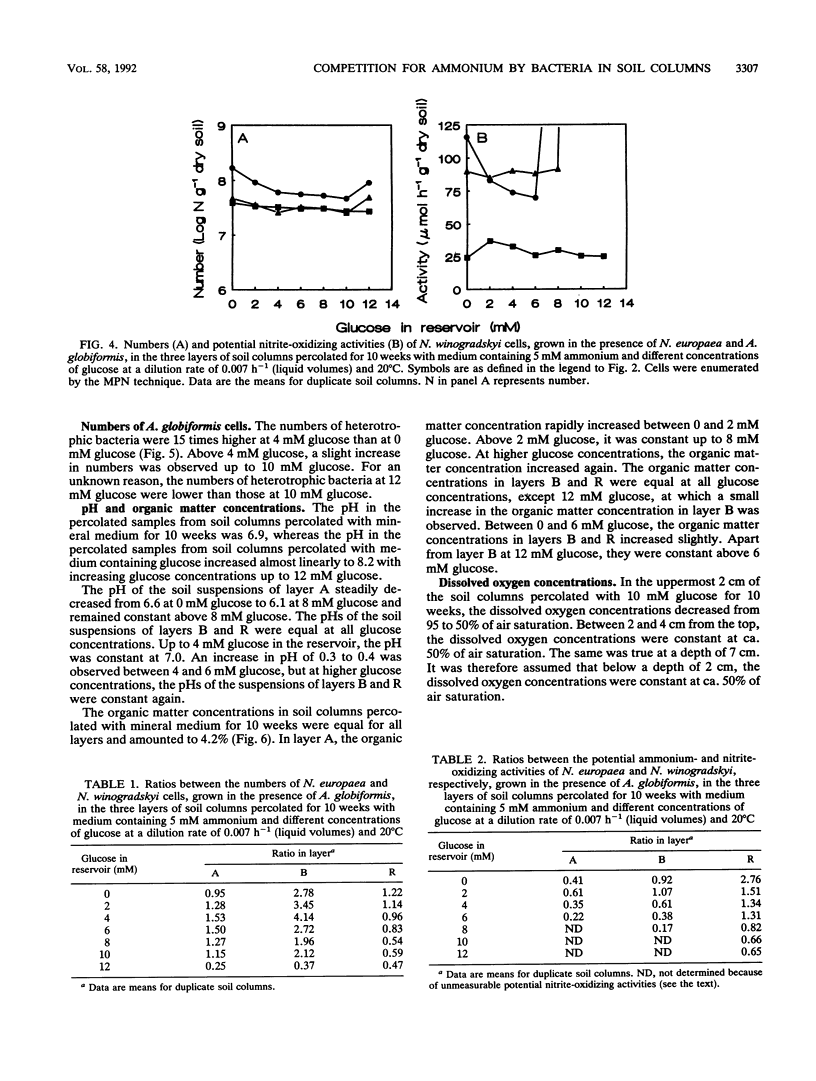
PDF

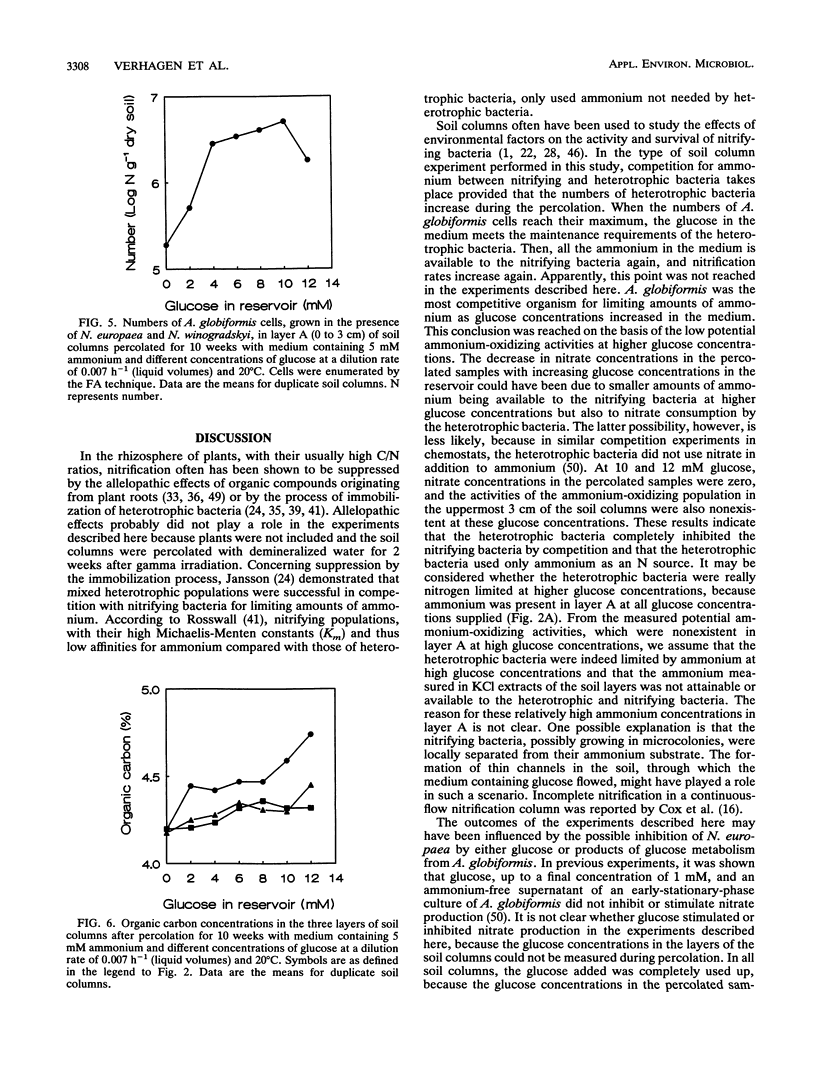



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belser L. W., Mays E. L. Specific inhibition of nitrite oxidation by chlorate and its use in assessing nitrification in soils and sediments. Appl Environ Microbiol. 1980 Mar;39(3):505–510. doi: 10.1128/aem.39.3.505-510.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser L. W., Mays E. L. Use of nitrifier activity measurements to estimate the efficiency of viable nitrifier counts in soils and sediments. Appl Environ Microbiol. 1982 Apr;43(4):945–948. doi: 10.1128/aem.43.4.945-948.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belser L. W., Schmidt E. L. Inhibitory effect of nitrapyrin on three genera of ammonia-oxidizing nitrifiers. Appl Environ Microbiol. 1981 Mar;41(3):819–821. doi: 10.1128/aem.41.3.819-821.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock E. Growth of nitrobacter in the presence of organic matter. II. Chemoorganotrophic growth of Nitrobacter agilis. Arch Microbiol. 1976 Jul;108(3):305–312. doi: 10.1007/BF00454857. [DOI] [PubMed] [Google Scholar]
- Clark C., Schmidt E. L. Growth response of Nitrosomonas europaea to amino acids. J Bacteriol. 1967 Apr;93(4):1302–1308. doi: 10.1128/jb.93.4.1302-1308.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. K., Knowles R. Inhibition of chemoautotrophic nitrification by sodium chlorate and sodium chlorite: a reexamination. Appl Environ Microbiol. 1983 Apr;45(4):1178–1182. doi: 10.1128/aem.45.4.1178-1182.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENSEN H. L. Effect of organic compounds on nitrosomonas. Nature. 1950 Jun 17;165(4207):974–974. doi: 10.1038/165974a0. [DOI] [PubMed] [Google Scholar]
- Powell S. J., Prosser J. I. Inhibition of ammonium oxidation by nitrapyrin in soil and liquid culture. Appl Environ Microbiol. 1986 Oct;52(4):782–787. doi: 10.1128/aem.52.4.782-787.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe R., Todd R., Waide J. Microtechnique for most-probable-number analysis. Appl Environ Microbiol. 1977 Mar;33(3):675–680. doi: 10.1128/aem.33.3.675-680.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmüller W., Bock E. Growth of Nitrobacter in the presence of organic matter. I. Mixotrophic growth. Arch Microbiol. 1976 Jul;108(3):299–304. doi: 10.1007/BF00454856. [DOI] [PubMed] [Google Scholar]
- Taylor P. A., leB Williams P. J. Theoretical studies on the coexistence of competing species under continuous-flow conditions. Can J Microbiol. 1975 Jan;21(1):90–98. doi: 10.1139/m75-013. [DOI] [PubMed] [Google Scholar]
- Verhagen F. J., Laanbroek H. J. Competition for Ammonium between Nitrifying and Heterotrophic Bacteria in Dual Energy-Limited Chemostats. Appl Environ Microbiol. 1991 Nov;57(11):3255–3263. doi: 10.1128/aem.57.11.3255-3263.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward B. B., Perry M. J. Immunofluorescent Assay for the Marine Ammonium-Oxidizing Bacterium Nitrosococcus oceanus. Appl Environ Microbiol. 1980 Apr;39(4):913–918. doi: 10.1128/aem.39.4.913-918.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H., Klinzing G., Blanch H. W. Competition for mixed substrates by microbial populations. Biotechnol Bioeng. 1977 Aug;19(8):1193–1210. doi: 10.1002/bit.260190809. [DOI] [PubMed] [Google Scholar]


