Abstract
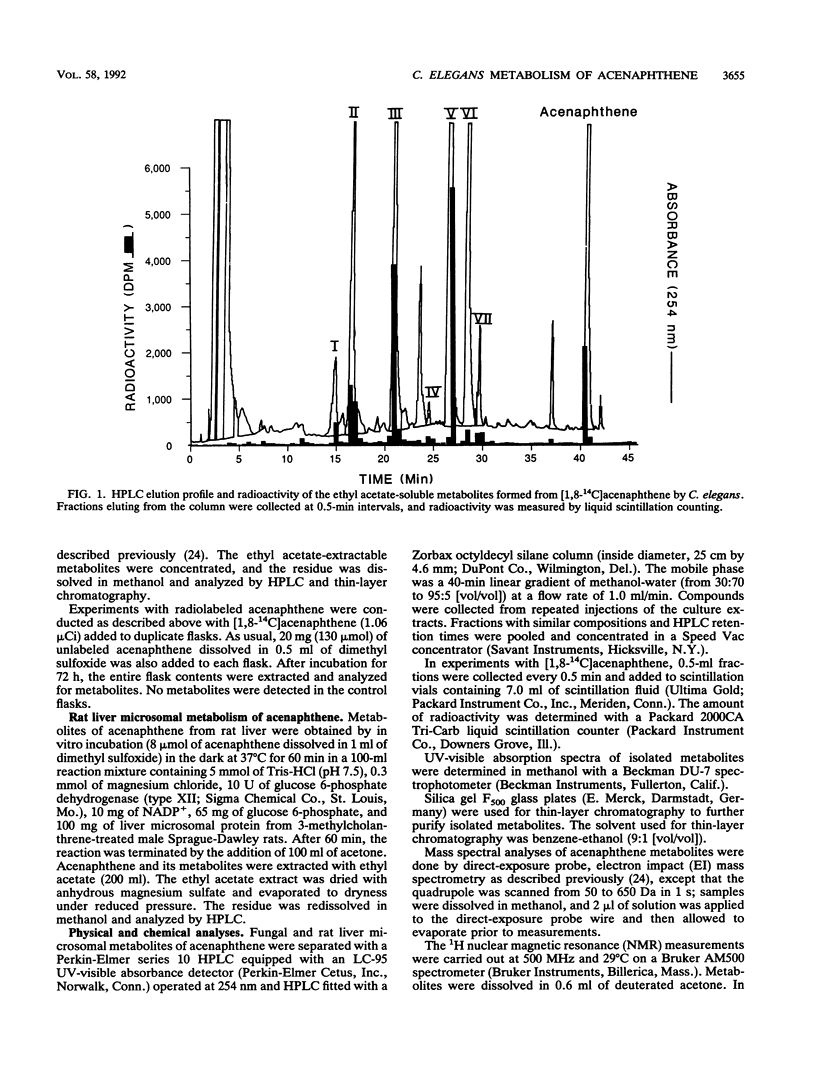
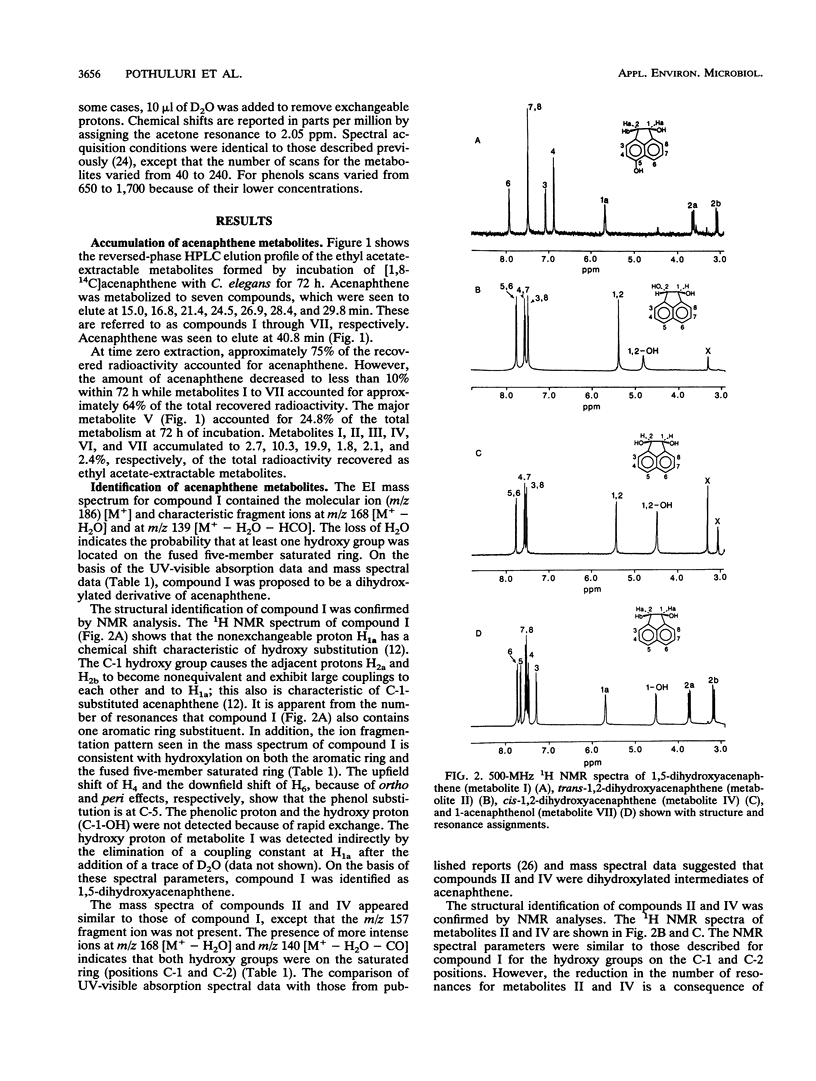
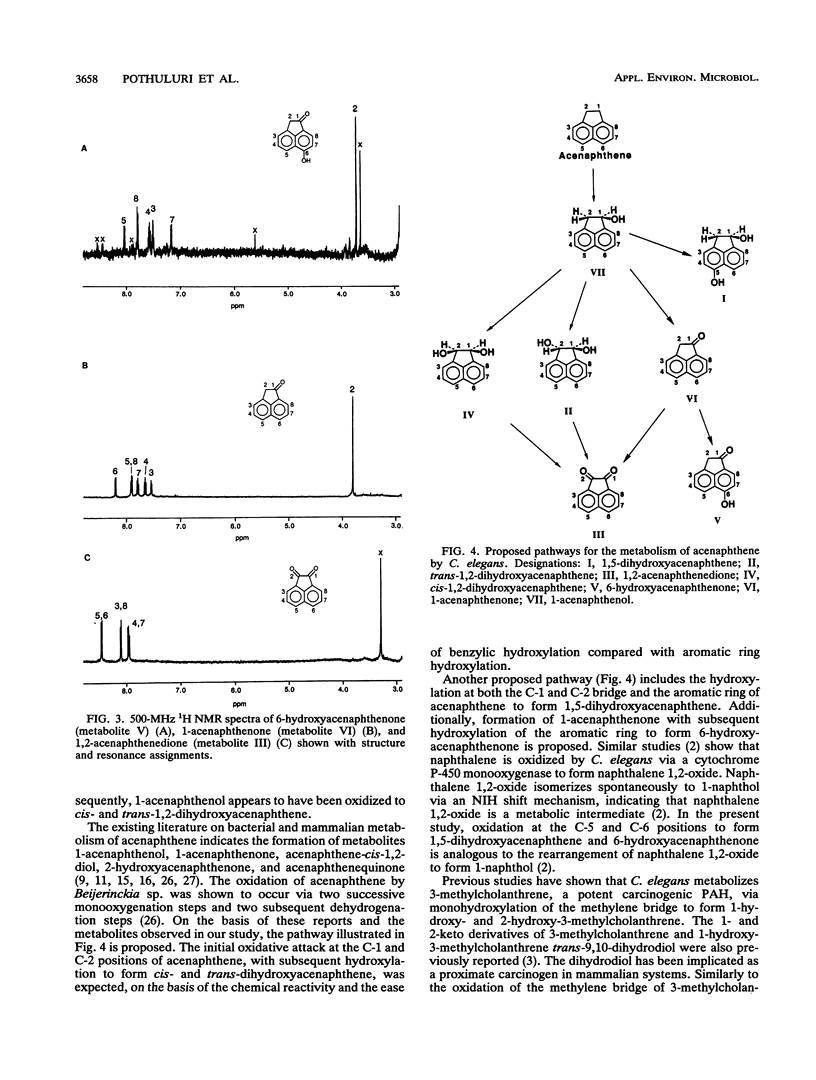
The filamentous fungus Cunninghamella elegans ATCC 36112 metabolized within 72 h of incubation approximately 64% of the [1,8-14C]acenaphthene added. The radioactive metabolites were extracted with ethyl acetate and separated by thin-layer chromatography and reversed-phase high-performance liquid chromatography. Seven metabolites were identified by 1H nuclear magnetic resonance, UV, and mass spectral techniques as 6-hydroxyacenaphthenone (24.8%), 1,2-acenaphthenedione (19.9%), trans-1,2-dihydroxyacenaphthene (10.3%), 1,5-dihydroxyacenaphthene (2.7%), 1-acenaphthenol (2.4%), 1-acenaphthenone (2.1%), and cis-1,2-dihydroxyacenaphthene (1.8%). Parallel experiments with rat liver microsomes indicated that the major metabolite formed from acenaphthene by rat liver microsomes was 1-acenaphthenone. The fungal metabolism of acenaphthene was similar to bacterial and mammalian metabolism, since the primary site of enzymatic attack was on the two carbons of the five-member ring.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLEARY G. J. Discreet separation of polycyclic hydrocarbons in air borne particulates usin very long alumina columns. J Chromatogr. 1962 Oct;9:204–215. doi: 10.1016/s0021-9673(00)80761-x. [DOI] [PubMed] [Google Scholar]
- Cairns M. A., Nebeker A. V. Toxicity of acenaphthene and isophorone to early life stages of fathead minnows. Arch Environ Contam Toxicol. 1982 Nov;11(6):703–707. doi: 10.1007/BF01059158. [DOI] [PubMed] [Google Scholar]
- Cerniglia C. E., Althaus J. R., Evans F. E., Freeman J. P., Mitchum R. K., Yang S. K. Stereochemistry and evidence for an arene oxide-NIH shift pathway in the fungal metabolism of naphthalene. Chem Biol Interact. 1983 Apr-May;44(1-2):119–132. doi: 10.1016/0009-2797(83)90134-5. [DOI] [PubMed] [Google Scholar]
- Cerniglia C. E., Dodge R. H., Gibson D. T. Fungal oxidation of 3-methylcholanthrene: formation of proximate carcinogenic metabolites of 3-methylcholanthrene. Chem Biol Interact. 1982 Jan;38(2):161–173. doi: 10.1016/0009-2797(82)90037-0. [DOI] [PubMed] [Google Scholar]
- Cerniglia C. E., Kelly D. W., Freeman J. P., Miller D. W. Microbial metabolism of pyrene. Chem Biol Interact. 1986 Feb;57(2):203–216. doi: 10.1016/0009-2797(86)90038-4. [DOI] [PubMed] [Google Scholar]
- Cerniglia C. E., Miller D. W., Yang S. K., Freeman J. P. Effects of a fluoro substituent on the fungal metabolism of 1-fluoronaphthalene. Appl Environ Microbiol. 1984 Aug;48(2):294–300. doi: 10.1128/aem.48.2.294-300.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond E. C., Callaghan P., Hopkins R. P. Metabolic dehydrogenation of cis- and trans-acenaphthene-1,2-diol. Xenobiotica. 1972 Nov;2(6):529–538. doi: 10.3109/00498257209111081. [DOI] [PubMed] [Google Scholar]
- Grimmer G., Böhnke H., Glaser A. Investigation on the carcinogen burden by air pollution in man. XV. Polycyclic aromatic hydrocarbons in automobile exhaust gas--an inventory. Zentralbl Bakteriol Orig B. 1977 May;164(3):218–234. [PubMed] [Google Scholar]
- Hopkins R. P. Microsomal ring-fission of cis- and trans-acenaphthene-1,2-diol. Biochem J. 1968 Jul;108(4):577–582. doi: 10.1042/bj1080577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins R. P., Young L. Biochemical studies of toxic agents. Metabolic ring-fission of cis- and trans-acenaphthene-1,2-diol. Biochem J. 1966 Jan;98(1):19–24. doi: 10.1042/bj0980019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihelcic J. R., Luthy R. G. Microbial degradation of acenaphthene and naphthalene under denitrification conditions in soil-water systems. Appl Environ Microbiol. 1988 May;54(5):1188–1198. doi: 10.1128/aem.54.5.1188-1198.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millner G. C., Fu P. P., Cerniglia C. E. Microbial transformation of 6-nitrobenzo[a]pyrene. J Toxicol Environ Health. 1986;19(4):519–530. doi: 10.1080/15287398609530949. [DOI] [PubMed] [Google Scholar]
- Pothuluri J. V., Freeman J. P., Evans F. E., Cerniglia C. E. Fungal transformation of fluoranthene. Appl Environ Microbiol. 1990 Oct;56(10):2974–2983. doi: 10.1128/aem.56.10.2974-2983.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothuluri J. V., Heflich R. H., Fu P. P., Cerniglia C. E. Fungal metabolism and detoxification of fluoranthene. Appl Environ Microbiol. 1992 Mar;58(3):937–941. doi: 10.1128/aem.58.3.937-941.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schocken M. J., Gibson D. T. Bacterial oxidation of the polycyclic aromatic hydrocarbons acenaphthene and acenaphthylene. Appl Environ Microbiol. 1984 Jul;48(1):10–16. doi: 10.1128/aem.48.1.10-16.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons C. J., Hopkins R. P., Callaghan P. Metabolic oxidation of acenaphthen-1-ol. Xenobiotica. 1973 Oct;3(10):633–642. doi: 10.3109/00498257309151587. [DOI] [PubMed] [Google Scholar]
- Sutherland J. B. Detoxification of polycyclic aromatic hydrocarbons by fungi. J Ind Microbiol. 1992 Jan;9(1):53–61. doi: 10.1007/BF01576368. [DOI] [PubMed] [Google Scholar]


