Abstract
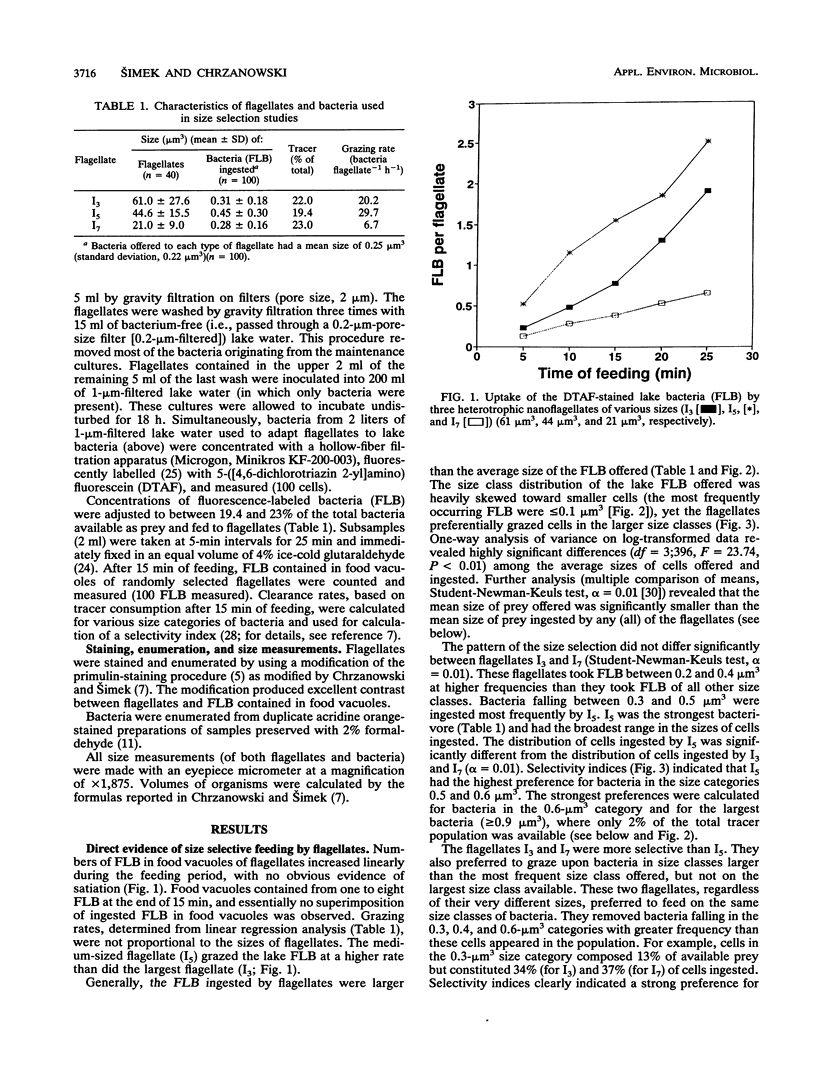
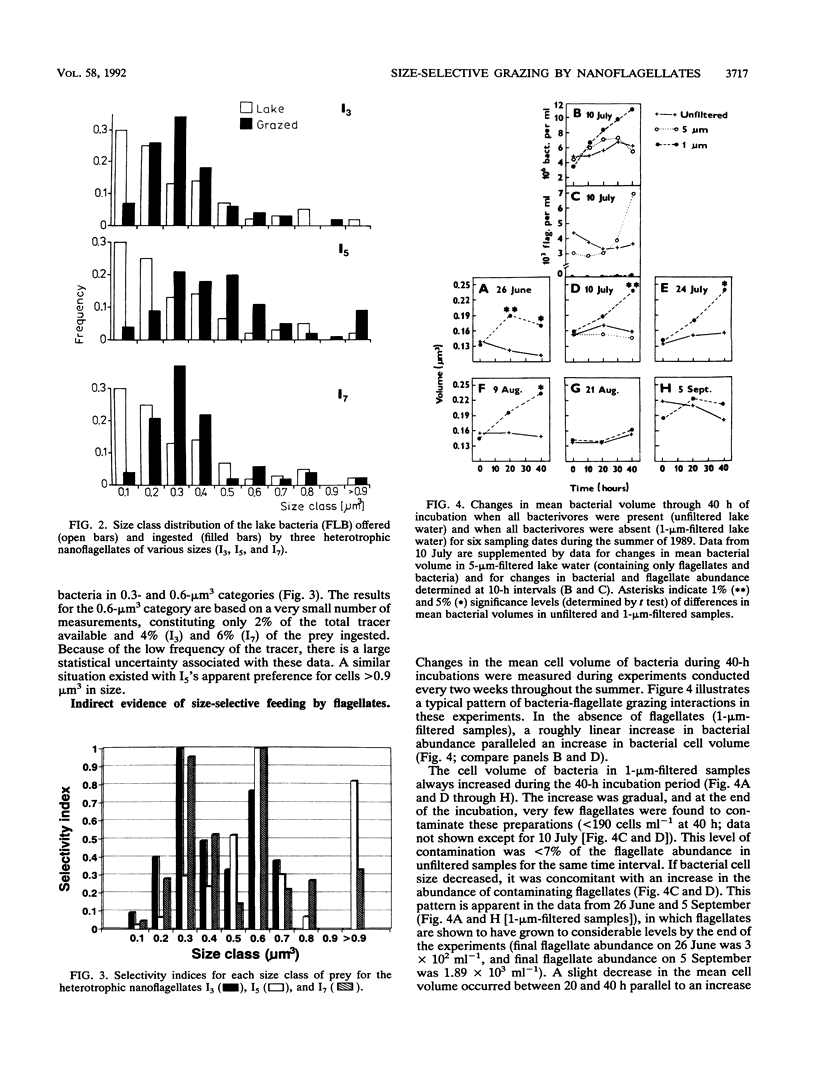
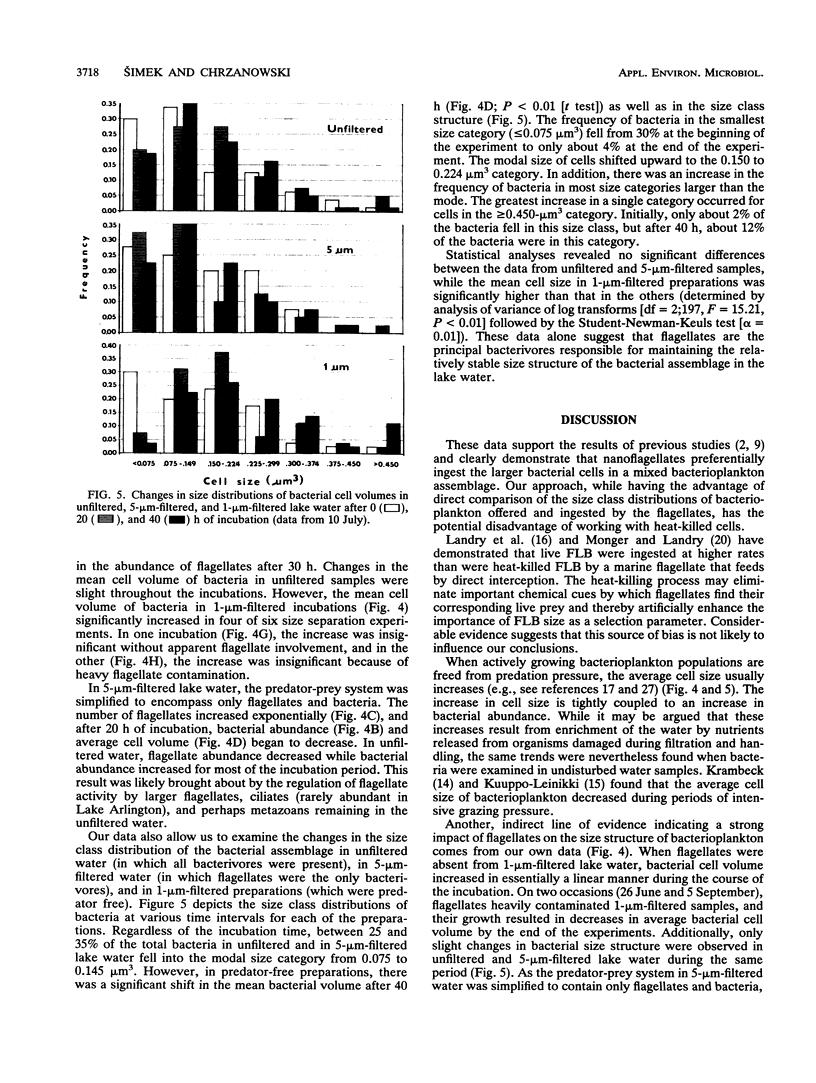
Size-selective grazing of three heterotrophic nanoflagellates (with cell sizes of 21, 44, and 66 μm3) isolated from Lake Arlington, Texas was examined by using a natural mixture of fluorescence labelled lake bacteria. Sizes of ingested bacteria in food vacuoles were directly measured. Larger bacterial cells were ingested at a frequency much higher than that at which they occurred in the assemblage, indicating preferential flagellate grazing on the larger size classes within the lake bacterioplankton. Water samples were collected biweekly from June through September, 1989, fractionated by filtration, and incubated for 40 h at in situ temperatures. The average bacterial size was always larger in water which was passed through 1-μm-pore-size filters (1-μm-filtered water) (which was predator free) than in 5-μm-filtered water (which contained flagellates only) or in unfiltered water (in which all bacterivores were present). The increase of bacterial-cell size in 1-μm-filtered water was caused by a shift in the size structure of the bacterioplankton population. Larger cells became more abundant in the absence of flagellate grazing.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloem J., Ellenbroek F. M., Bär-Gilissen M. J., Cappenberg T. E. Protozoan grazing and bacterial production in stratified lake vechten estimated with fluorescently labeled bacteria and by thymidine incorporation. Appl Environ Microbiol. 1989 Jul;55(7):1787–1795. doi: 10.1128/aem.55.7.1787-1795.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron D. A. Technique for enumeration of heterotrophic and phototrophic nanoplankton, using epifluorescence microscopy, and comparison with other procedures. Appl Environ Microbiol. 1983 Aug;46(2):491–498. doi: 10.1128/aem.46.2.491-498.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez J. M., Sherr E. B., Sherr B. F. Size-selective grazing on bacteria by natural assemblages of estuarine flagellates and ciliates. Appl Environ Microbiol. 1990 Mar;56(3):583–589. doi: 10.1128/aem.56.3.583-589.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr B. F., Sherr E. B., Fallon R. D. Use of monodispersed, fluorescently labeled bacteria to estimate in situ protozoan bacterivory. Appl Environ Microbiol. 1987 May;53(5):958–965. doi: 10.1128/aem.53.5.958-965.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr B. F., Sherr E. B., McDaniel J. Effect of protistan grazing on the frequency of dividing cells in bacterioplankton assemblages. Appl Environ Microbiol. 1992 Aug;58(8):2381–2385. doi: 10.1128/aem.58.8.2381-2385.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


