Abstract
Many bacterial pathogens have the ability to induce apoptosis in their hosts. It was previously shown that Nocardia asteroides strain GUH-2, a Gram-positive facultatively intracellular pathogen, is capable of inducing the apoptotic death of dopaminergic cells in the murine brain and in PC12 cells, a rat cell line. In this study, the apoptosis-inducing potential of N. asteroides GUH-2 was further explored using HeLa cells, a human epithelial cell line. HeLa cells were incubated for 5 hours with live nocardiae, heat-killed bacteria, or unconcentrated nocardial culture filtrate, and changes to the cells were monitored. Consistent with the previous studies, N. asteroides GUH-2 induced DNA fragmentation and apoptosis in HeLa cells. Caspase activation and disruption of the mitochondrial membrane potential were also investigated to determine their roles in the induction of cell death. In all these experiments, significant changes were only induced by live nocardiae. A recent publication demonstrated that systemic administration of proteasome inhibitors can induce a Parkinsonian syndrome in rats that includes intraneuronal inclusions and characteristic behavioral alterations. Similar effects have been observed in mice and monkeys infected with N. asteroides GUH-2. In addition, some reports have shown that proteasome inhibition causes apoptotic death of affected cells. We therefore investigated the ability of N. asteroides GUH-2 to inhibit proteasome activity. Proteasome activity was significantly reduced, suggesting that this mechanism may be involved in the induction of apoptosis by these bacteria.
Keywords: Apoptosis, proteasome endopeptidase complex, Nocardia, caspases, Parkinson disease, HeLa cells
1. Introduction
Apoptosis, or programmed cell death, is essential for the proper development and health of multicellular eukaryotes, allowing for safe elimination of senescent cells, regulation of the immune system, and proper development of the organs [14, 23, 33]. During apoptosis, a characteristic signaling cascade occurs that eventually leads to disruption of the nucleus and blebbing of the cell into small apoptotic bodies that are then phagocytosed by surrounding cells [16, 49]. Some pathogenic microorganisms can interfere with this tightly controlled system and cause inopportune apoptotic death of cells in their eukaryotic hosts as part of the disease process. Bacterial induced apoptosis is a widespread phenomenon that has been reported for a variety of species [34].
The genus Nocardia comprises a group of obligately aerobic, Gram-positive, filamentous bacteria. Nocardiae are facultative intracellular pathogens that infect a wide range of species, including humans, and can cause a variety of disorders dependent on the immunocompetence of the host and the organ infected [3]. The mechanisms that nocardiae utilize to cause disease are not completely understood. However, our earlier research indicated that highly virulent N. asteroides strain GUH-2 causes apoptosis in the murine brain and in PC12 cells, a dopaminergic cell line. Apoptotic cell death was observed using assays to identify cells containing fragmented DNA or exhibiting plasma membrane rearrangement [43]. Similar effects have also been observed using culture filtrate from GUH-2. PC12 cell apoptosis was demonstrated via morphological changes and DNA fragmentation after only a 5-minute exposure to the filtrate [30]. Other cell lines also were similarly affected [6].
As nocardial infections most often occur in the lung, an epithelial cell-lined organ, we investigated the apoptosis-inducing ability of N. asteroides GUH-2 towards HeLa cells, an immortalized epithelial cell line commonly used in studies of bacteria-host interactions [1, 9, 31, 45, 46]. We expanded our previous investigations by examining the involvement of caspases (cysteine aspartate proteases) in nocardial induced cell death. We also examined the effect of bacteria on HeLa cell mitochondria, since secreted bacterial toxins have been implicated in triggering apoptosis via this organelle [22, 24], and previous studies have shown that some secreted product of nocardiae has the ability to cause apoptosis [6, 30]. Along with these studies, we explored the requirements for induction of apoptotic host cell death by comparing the effects of live bacteria, heat-killed nocardiae, and unconcentrated culture filtrate.
In addition, we investigated inhibition of the proteasome as a potential mechanism for the induction of apoptosis. The proteasome is a multiunit enzyme complex, which is responsible for the degradation of misfolded or unneeded proteins [44]. Recently, McNaught et al. reported that proteasome inhibitors injected into rats can induce a Parkinsonian syndrome that includes intraneuronal inclusions, bradykinesia, rigidity, tremor, and an abnormal posture [32]. Epoxomicin, one of the inhibitors used, is naturally produced by a soil-dwelling aerobic actinomycete [21]. N. asteroides GUH-2 and related bacteria have also been found to induce Parkinsonism in mice, following intravenous administration [13, 28]. In both Parkinson’s disease and Lewy body dementia, there is an accumulation of ubiquinated protein and alpha synuclein fibrils that form intraneuronal inclusions known as Lewy bodies [17]. The formation of these structures has been linked to inhibition of the ubiquitin-proteasome system [2, 7, 36]. Similar intraneuronal inclusions have been observed within the brains of mice or monkeys infected with neuroinvasive nocardiae [8, 13, 28]. Furthermore, in experiments utilizing nocardial culture filtrates to induce dopamine depletion and apoptosis of PC12 cells, Loeffler et al. localized the activity within the sub-3-kDa fraction [30]. Treatment with heat, proteases, and chloroform had no effect on the activity suggesting that some small, stable molecule was responsible for the observed effects.
Our experiments in the current study revealed that mitochondrial disruption and caspases are involved in the pathways of nocardial induced cell death. Additionally, the present study revealed that neither heat-killed bacteria, nor unconcentrated culture filtrate had any significant effect on HeLa cells. Only live nocardiae were able to cause apoptosis. The involvement of a secreted proteasome inhibitor was also implicated in experiments using cultured cells and purified proteasome.
2. Materials and Methods
2.1 Preparation of bacteria and unconcentrated culture filtrate
Nocardia asteroides strain GUH-2 was grown in brain heart infusion broth (BHI; Difco) at 37°C with constant agitation at 150 RPM. After approximately 18 hours of incubation, the bacteria were centrifuged at 50 x g to remove aggregates and the optical density at 580 nm was measured to give an estimate of the bacterial concentration. Actual concentrations were determined by plating serial dilutions of the culture on BHI agar. Heat-killed bacteria were prepared by heating cultures at 65°C for 2 hours. Culture filtrate was prepared by passing the culture through a 0.2 μm syringe filter.
2.2 Preparation of HeLa cells
HeLa cells (American Type Culture Collection) were cultured in 25- or 75-cm2 tissue culture flasks at 37°C with 5% CO2 in minimal essential medium supplemented with 10% fetal bovine serum, 1 mM sodium pyruvate, 100 μM non-essential amino acids, 100 U/ml penicillin, 100 μg/ml streptomycin, and 250 μg/ml amphotericin B (Gibco) and split when near confluence. As a first step in the assays described below, the cells were collected, washed, and plated in medium without antibiotics.
2.3 Detection of DNA fragmentation
HeLa cells were plated in 6-well tissue culture plates (106 cells per well) and incubated overnight, some with 0.5 μM staurosporine as a positive control for apoptosis. Live or heat-killed nocardiae (at an approximate MOI of 5:1) or unconcentrated culture filtrate (in an equivalent volume) was then added to appropriate wells and the cells were incubated at 37°C. The standard TUNEL assay was then performed [43]. Briefly, following incubation the cells were harvested, washed, and incubated with labeling solution containing fluorescently tagged nucleotides and terminal deoxyribonucleotidyltransferase for 90 minutes according to the manufacturer's instructions (Calbiochem). Cells were analyzed by flow cytometry and the mean fluorescence intensity of each sample was determined. To visualize apoptotic morphology, some labeled cells were additionally treated with 100 U/ml RNase A plus 12.5 μg/ml PI, to stain cell nuclei (modified from [41]), and were then spun onto slides, mounted, and viewed by confocal microscopy.
2.4 Visualization of nocardial adherence and invasion
HeLa cells were plated on 8-chamber tissue culture slides (5x103 cells per well) and incubated overnight. Live or heat-killed N. asteroides GUH-2 were non-specifically labeled with 1 mg/ml fluorescein isothiocyanate for 30 minutes and then added to appropriate wells. The cells were incubated for 2 hours and then washed to remove non-adherent bacteria. Cells were fixed and permeablized, and cellular actin was then labeled with rhodamine-phalloidin. HeLa cells and adherent nocardiae were visualized by confocal microscopy.
2.5 In situ labeling of active caspases
HeLa cells were plated in 24-well tissue culture plates (3x105 cells per well) and incubated overnight, some with 0.5 μM staurosporine as a positive control. Live or heat-killed nocardiae (at an approximate MOI of 5:1) or unconcentrated culture filtrate (in an equivalent volume) was then added to appropriate wells and the cells were incubated for 5 hours at 37°C. Following incubation, the cells were harvested, washed, and resuspended in medium containing the fluorescently tagged pan-caspase inhibitor FAM-VAD-FMK according to the manufacturer's instructions (Chemicon). Cells were incubated an additional hour, washed, and counterstained with propidium iodide (PI). Cells were analyzed on a FACScan flow cytometer (Becton Dickinson) using Cellquest software. The mean fluorescence intensity of the fluorescein channel was determined in cells that maintained an intact plasma membrane, as demonstrated by exclusion of PI.
2.6 Measurement of caspase 3 activity
HeLa cells were plated in 6-well tissue culture plates and incubated as described above. Nocardiae or culture filtrate was then added and the cells were incubated for 5 hours. Following incubation, the cells were harvested, washed, and lysed by rapid freezing and thawing. The protein concentration of each sample was measured using the DC Protein Assay (BioRad). Caspase activity was measured by monitoring the proteolytic cleavage of p-nitroaniline (pNA) from the caspase-3 substrate peptide, Ac-DEVD-pNA, via a kinetic microplate assay, according to the manufacturer's instructions (Calbiochem). Relative activity per μg protein was calculated from the slope of the line obtained by graphing optical density versus time, and the data were then normalized by dividing by the negative control values.
2.7 Monitoring of mitochondrial membrane potential
HeLa cells were plated in 6-well tissue culture plates and incubated as described above. Live nocardiae or sterile broth was then added and the cells were incubated for 5 hours. Following incubation, the cells were harvested, washed, and incubated 10 minutes with 20 nM 3,3′-dihexyloxacarbocyanine (DiOC6(3)), a fluorescent probe that accumulates within mitochondria having intact membrane potential, and 2 μg/ml PI. As a positive control for mitochondrial depolarization, some cells were coincubated with 200 μM carbonyl cyanide m-chlorophenylhydrazone (CCCP), which disrupts the mitochondrial membrane potential. The cells were analyzed on a FACScan flow cytometer using Cellquest software and the mean fluorescence was determined.
2.8 Apoptosis assays using concentrated culture filtrate
N. asteroides GUH-2 was grown in α-MEM (Omega Scientific) for 19.5 hours at 37°C with constant agitation at 150 RPM. Following incubation, the culture was filtered through a 0.22 μm membrane by vacuum to remove all cells. This crude culture filtrate was then further purified using a Centricon Plus-80 centrifugal filter device (Amicon) to remove components larger than 8 kDa. Finally, the fractionated culture filtrate was concentrated 10-fold using a SpeedVac concentrator (Savant). This concentrated culture filtrate was then tested for apoptosis-inducing activity. The assays described above for detecting in situ activation of caspases and for measuring mitochondrial depolarization were performed with one modification. Instead of nocardiae, HeLa cells were exposed for 5 hours to a 1:10 dilution of the 10-fold concentrated culture filtrate, giving a final 1X concentration. Appropriate control wells were also incubated. Following exposure, the procedures were completed as described above.
2.9 Measurement of proteasome activity in cell lysates
HeLa cells were plated in 6-well plates and incubated overnight. The cells were then incubated for 5 hours with either 0.5 μM epoxomicin (a known proteasome inhibitor) as a positive control [21], nocardiae, or sterile culture medium. Following incubation, the cells were harvested, washed, and lysed by rapid freezing and thawing four times. Particulate components were removed by centrifugation at 10,000 x g, and the protein concentration of each sample was measured using the DC Protein Assay (BioRad). Proteasome activity was measured using a protocol modified from Elliott et al. [15]. Cell lysates were incubated with one of three different fluorogenic proteasome peptide substrates (Peptides International): Suc-Leu-Leu-Val-Tyr-MCA to monitor chymotrypsin-like activity, Boc-Leu-Arg-Arg-MCA to measure trypsin-like activity, or Z-Leu-Leu-Glu-MCA to follow peptidylglutamyl peptide hydrolyzing (PGPH) activity. Individual wells of a black 96-well plate were filled with 80 μl assay buffer (50 mM HEPES, pH 7.4, 100 mM NaCl, 0.1% CHAPS, 10 mM DTT, 0.1 mM EDTA, 10% glycerol), 10 μl cleared lysate, and 10 μl substrate (50 μM final concentration). A SpectraMax M2 fluorometer (Molecular Devices) was used to determine enzyme activity by observing the increase of fluorescence over a 60-minute period, as the fluorogenic 7-amino-4-methylcoumarin (MCA) was cleaved from the substrate. The resulting curves were adjusted to account for differences in initial protein concentration, and all data were then normalized to give activities relative to the negative controls.
This experiment was also performed using PC12 cells grown in RPMI 1640 supplemented with 10% horse serum and 5% fetal bovine serum (Gibco). The cells were exposed to the agents described above and lysates were collected and assayed to measure proteasome activity.
2.10 Effect of concentrated culture filtrate on pure proteasome
To confirm that the proteasome was being inhibited, and not just other proteases, individual wells of a black 96-well plate were filled with 70 μl assay buffer, 10 μL (50 ng total) purified 20S proteasome (Boston Biochem), 10 μl concentrated culture filtrate or sterile α-MEM, and 10 μl substrate (50 μM final concentration). The increase of fluorescence over time was measured over one hour and normalized as described above.
2.11 Statistical analysis
Differences between groups were analyzed by one factor ANOVA followed by Tukey’s honestly significant different test or Dunnett's test for comparison of multiple groups to a control. Differences were declared significant at p<0.05.
3. Results
3.1. Detection of DNA fragmentation
Fragmentation of DNA and the orderly disintegration of the cell and its organelles are among the final steps in the apoptotic process. The cleavage of DNA was measured using the standard TUNEL assay, in which fluorescently labeled nucleotides are added to the 3′ ends of DNA fragments. To monitor the progression of apoptosis, a time course study was performed using live nocardiae. After 1, 3, or 6 hours of exposure the cells exposed to nocardiae were collected and analyzed. Fig. 1 indicates that significant DNA fragmentation was only detected after 6 hours. Since the TUNEL assay identifies cells in the final stages of apoptosis, all further experiments were performed with a 5-hour incubation to limit the chance of missing earlier events.
Fig. 1.
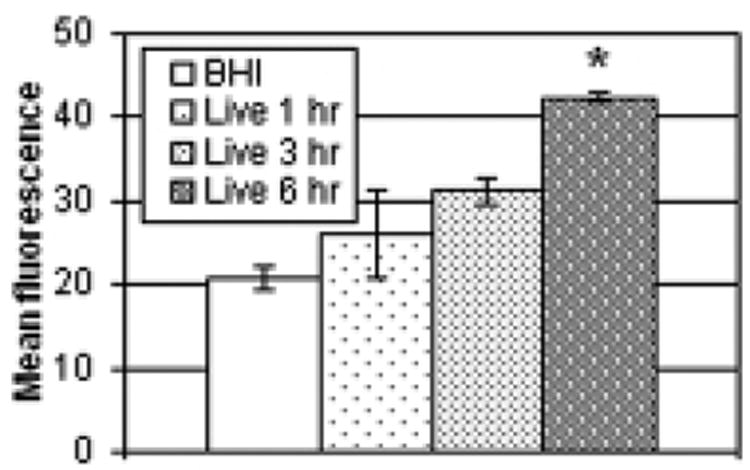
Time course study of DNA fragmentation. HeLa cells were exposed to live N. asteroides GUH-2. After 1, 3, and 6 hours of incubation, samples were collected and analyzed by the TUNEL assay to determine the level of DNA fragmentation. The graph indicates the mean fluorescence and standard error of each treatment group, with greater fluorescence indicative of greater DNA fragmentation. Asterisks indicate populations that were significantly different from the BHI control (p<0.05)
HeLa cells were incubated for 5 hours with either bacteria or unconcentrated culture filtrate. Fig. 2 illustrates the results of a representative experiment (n=3 per condition). Only live bacteria had a significant effect (mean fluorescence intensity of 23.88 vs. 8.55 for the BHI control), although killed bacteria induced greater fragmentation than did the culture filtrate.
Fig. 2.
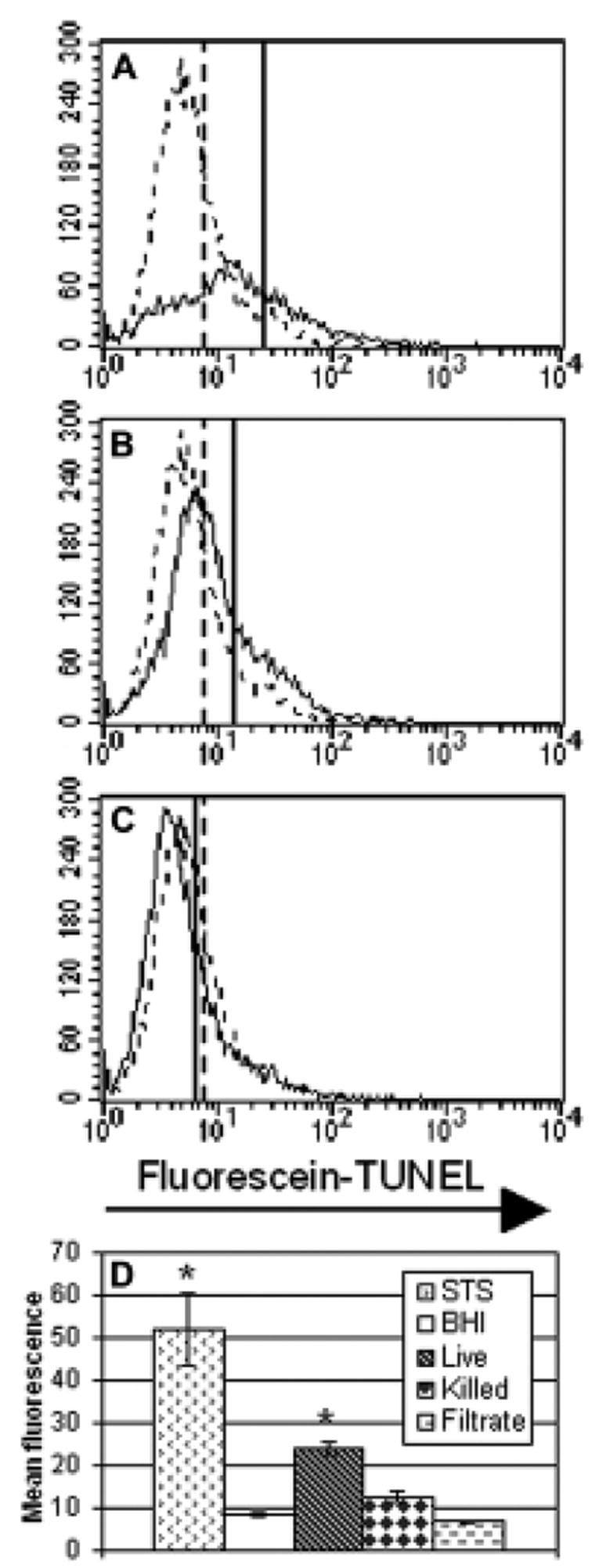
Induction of DNA fragmentation. Panels A, B, and C are representative histograms from a single experiment. In each, the experimental data are indicated with a solid line and a representative BHI control is shown as a dashed line for comparison. Vertical lines indicate the mean fluorescence of each sample. Panel A displays cells exposed to live GUH-2. Panel B focuses on the effect of heat-killed bacteria. Panel C looks at cells exposed to unconcentrated culture filtrate. Panel D is a bar graph summary of the results (n=3 for each condition). The bars indicate the mean fluorescence intensity of cells for each experimental group, with greater fluorescence indicating more DNA fragmentation. The bar labeled STS indicates the results of treatment with staurosporine, used as a positive control. Standard error is shown for each bar and the asterisks indicate a significant difference from the BHI control (p<0.01).
Microscopic observation was also used to confirm the presence of apoptotic morphological changes. Fig. 3 depicts cells dually treated with PI to stain the nucleus and by TUNEL to detect DNA fragmentation. Figs. 3A and 3B show negative and positive control cells, respectively. Figs. 3C and 3D show HeLa cells exposed to live GUH-2 that contained fragmented DNA. Nocardial filaments are seen among apoptotic HeLa cells in Fig. 3C. There is great morphological similarity between the positive control (Fig. 3B) and the cell shown in panel 3D.
Fig. 3.
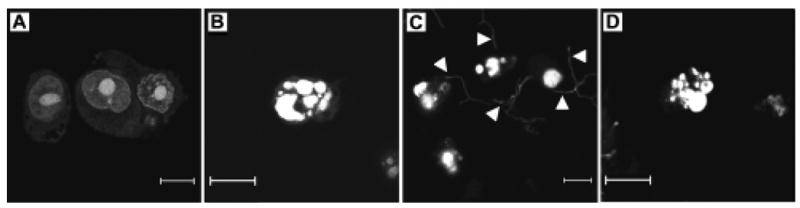
Nuclear alterations in HeLa cells. Panels show cells double stained with PI to indicate nuclear morphology (dull gray) and fluorescein-labeled nucleotides incorporated by the TUNEL procedure to indicate DNA fragmentation. Double-labeled cells appear bright in these images. Panel A shows negative control cells with no DNA fragmentation or condensation. Panel B is a positive control cell (incubated with staurosporine) with fragmented DNA and prototypical apoptotic nuclear morphology. Panels C and D show cells incubated with live N. asteroides GUH-2 for 5 hours. In panel C, nocardial filaments (indicated by arrowheads) non-specifically stained with PI are evident. The cell in panel D displays similar morphology to the positive control. The bar in each panel indicates a 10 μm distance.
3.2 Visualization of nocardial adherence
Virulent N. asteroides GUH-2 adhere to and invade many cell types. Fig. 4 demonstrates nocardial adherence to and invasion of HeLa cells. Nocardiae were stained non-specifically with fluorescein and were incubated with HeLa cells for 2 hours. HeLa cell actin was labeled with rhodamine-phalloidin. Live bacteria both adhered to (Fig. 4A) and invaded (Fig. 4B) HeLa cells, while heat-killed nocardiae could only adhere (Fig. 4C).
Fig. 4.
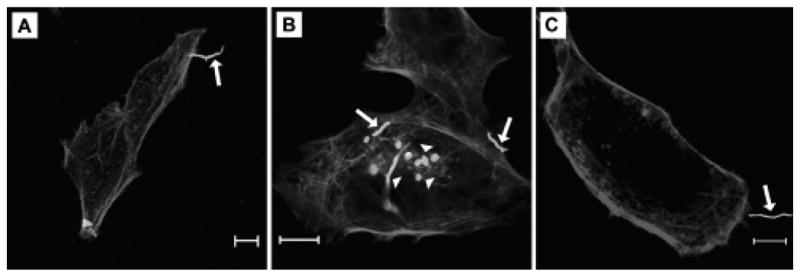
Bacterial adherence to and invasion of HeLa cells. Panels demonstrate nocardial adherence to and invasion of HeLa cells. HeLa cells were incubated with fluorescein-labeled bacteria for 2 hours and then washed and counterstained with rhodamine-phalloidin. In these panels, GUH-2 appears bright and HeLa cell actin appears dull. Panels A and B show live N. asteroides GUH-2 adhering by the filament tip and invading HeLa cells. In panel C, adherence by a heat-killed bacterium is shown. Arrows indicate bacteria adhered to cells. Arrowheads label nocardiae that appear to be within cells. The bar in each panel indicates a 10 μm distance.
3.3 Nocardia asteroides GUH-2 induces caspase activation
Caspase activation is a hallmark of many pathways of apoptosis. To monitor this event, HeLa cells were cultured with nocardiae or unconcentrated filtrate for 5 hours and then incubated with a fluorescent pan-caspase inhibitor that binds irreversibly to the functional site of activated caspases. Fig. 5 illustrates the results of a representative experiment. Only live bacteria significantly increased the activation of caspases (190.74 mean fluorescence vs. 108.69 for the BHI control). Although heat-killed bacteria did not have a significant effect, it may be relevant that in every repetition of this experiment the percentage of labeled cells exposed to killed bacteria was higher than that of cells exposed to unconcentrated culture filtrate.
Fig. 5.
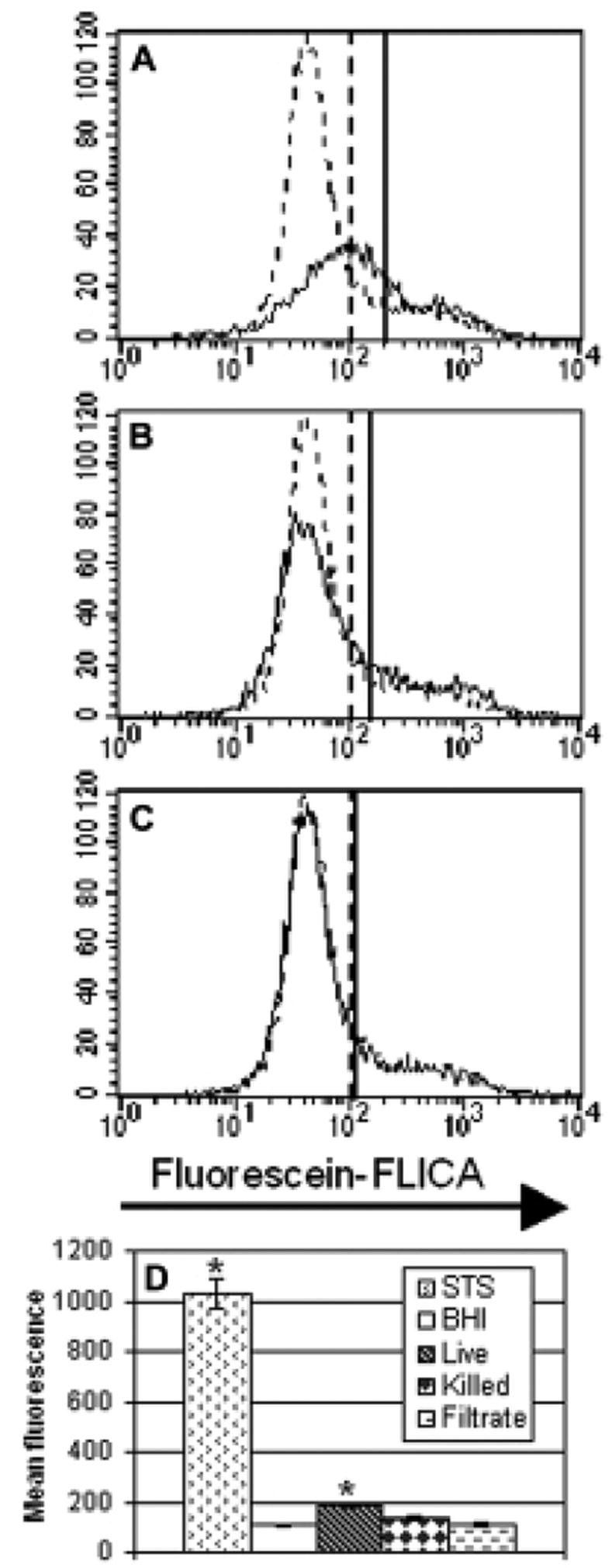
Caspase activation in HeLa cells following treatment. Panels A, B, and C are representative histograms from the same treatment groups as in Fig. 2. Experimental data are indicated with a solid line and the BHI control is shown as a dashed line. Prior to this display, the cells were gated to exclude cells that permitted entry of the PI label—indicative of a disruption of plasma membrane integrity. The vertical lines signify the mean fluorescence intensity in the fluorescein channel, with greater fluorescence indicating more caspase activation. Panel A displays cells exposed to live GUH-2. Panel B focuses on the effect of heat-killed bacteria. Panel C presents cells exposed to unconcentrated filtrate. Panel D is a bar graph summary of the results (n=4 for each condition), with bars indicating the average fluorescence of cells in each experimental group. The bar labeled STS indicates the results of treatment with staurosporine. Standard error is shown for each bar and the asterisks indicate a significant difference from the BHI control (p<0.01).
3.4 Caspase-3-like activity is increased in HeLa cells exposed to GUH-2
Caspase-3 is particularly vital in most described pathways of apoptosis. It is activated by a number of upstream initiator caspases and acts as a convergence point to trigger common downstream events. To determine its involvement here, cellular protein extracts from HeLa cells exposed to bacteria or unconcentrated culture filtrate were collected and caspase-3-like activity was determined by measuring the cleavage of the colorimetric substrate Ac-DEVD-pNA over time. Fig. 6 summarizes the results of eight experiments. As was observed for the caspase activation experiment described above, only live GUH-2 had a significant effect.
Fig. 6.
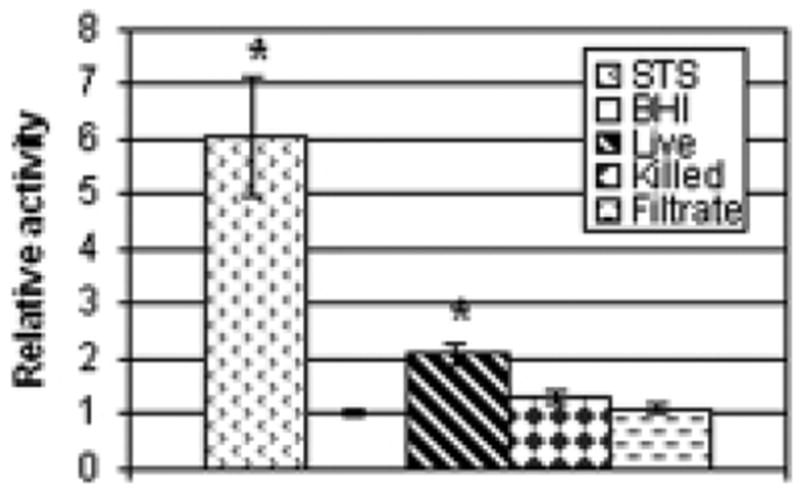
Effect of different treatments on caspase-3-like activity in HeLa cells. After a 5-hour incubation with staurosporine (STS), BHI, live GUH-2, heat-killed bacteria, or unconcentrated filtrate, cellular contents were extracted and assayed for caspase-3-like activity and protein concentration. Multiple experiments were performed to test these conditions, and activity was normalized for each by dividing by the BHI control values. Values from all experiments (n ranged from 12 to 24 per group) were then averaged for the graph. The bars indicate the average relative caspase-3-like activity for each group. Standard error is shown for each bar and the asterisks indicate a significant difference from the BHI control (p<0.01).
3.5 Disruption of mitochondrial potential
The intrinsic pathway of apoptosis involves signals mediated through the mitochondria. Following mitochondrial insult, there is a release of cytochrome c from the intermembrane space, which then complexes with and activates caspase-9. Release of this signaling protein is preceded by loss of mitochondrial potential, which can be monitored by incubating experimentally-treated cells with the fluorescent carbocyanine dye DiOC6(3). Following exposure of HeLa cells to live nocardiae or sterile culture medium, cells were briefly incubated with the probe and with the cell-impermeant dye PI. Fig. 7 shows the mean DiOC6(3) fluorescence of the various treatment groups on the cells that excluded the PI and therefore maintained membrane integrity. Mitochondrial potential was significantly decreased in cells exposed to live GUH-2.
Fig. 7.
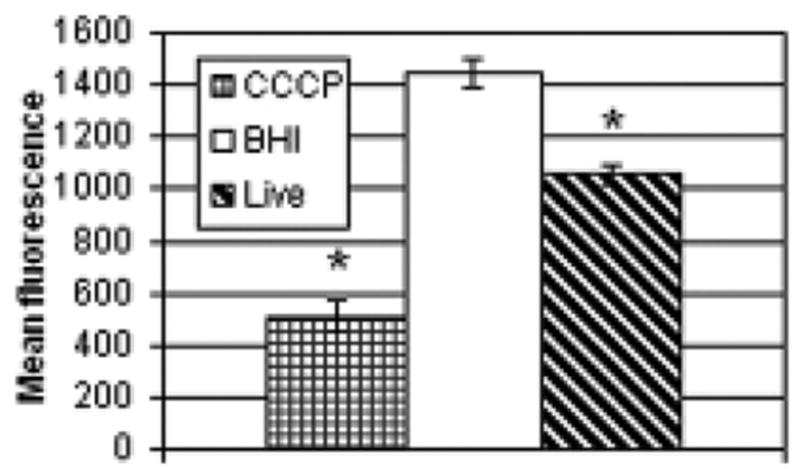
Mitochondrial potential in cells exposed to nocardiae. HeLa cells were incubated with live nocardiae or sterile BHI for 5 hours. Following exposure, cells were briefly incubated with DiOC6(3), which labels mitochondria with intact membrane potential, and with the cell-impermeant dye PI. Some cells were concurrently treated with CCCP, a disruptor of mitochondrial potential, as a positive control. Cellular fluorescence was determined by flow cytometry. The mean DiOC6(3) fluorescence of the cells (n 3 per group) that excluded the PI, and therefore maintained membrane integrity, is shown. Standard error is shown for each bar and the asterisks indicate a significant difference from the BHI control (p<0.01).
3.6 Apoptosis induced by concentrated culture filtrate
In the experiments reported above, it was noted that the culture filtrate had no effect in any of the assays. This finding contrasted with that of Loeffler et al. [30], which showed that culture filtrate alone was capable of triggering apoptotic death. In those experiments, PC12 cells were exposed to undiluted culture filtrate. To imitate those conditions, HeLa cells were incubated for 5 hours with 10-fold concentrated culture filtrate diluted 1:10. Following exposure, caspase activation and mitochondrial depolarization were measured. Fig. 9 shows the results of these experiments. In the in situ caspase labeling experiment (Fig. 8A), concentrated culture filtrate (CCF) significantly increased the level of activated caspases in the cells. The CCF also induced mitochondrial depolarization, depicted in Fig. 8B.
Fig. 9.
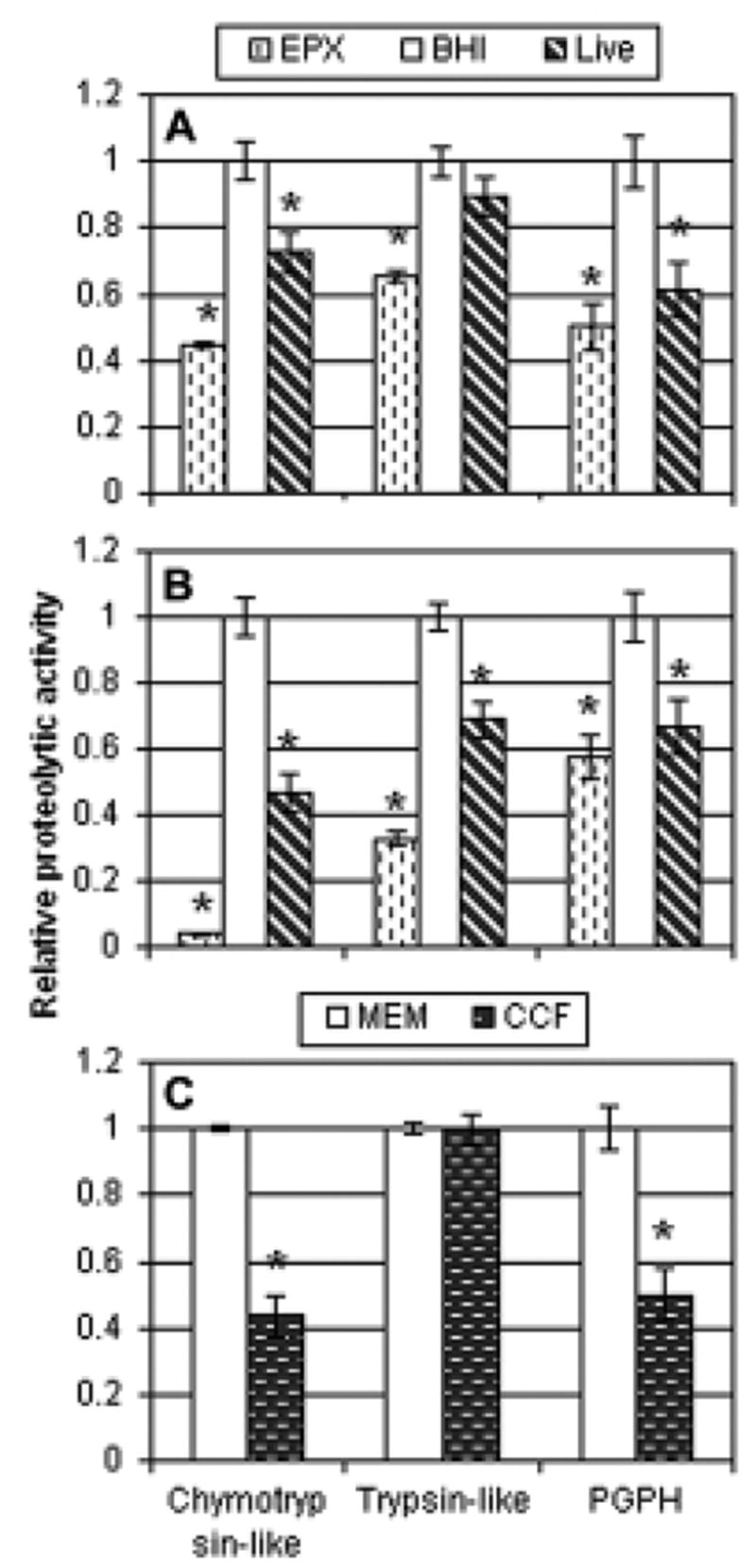
Proteasome inhibition observed in cell lysates and with purified proteasome. The relative proteolytic activities compared to the negative control are shown, with standard errors. For panels A and B, cells were incubated with 0.5 μM epoxomicin (EPX), Nocardia asteroides strain GUH-2 (GUH-2), or sterile culture medium (BHI). Panel A indicates effects on HeLa cells. Panel B shows effects on PC12 cells. For each panel, two experiments were performed (n=4 per group) and the data were normalized. All the normalized data for each cell type were then statistically analyzed and graphed. For panel C, purified rabbit 20S proteasome was incubated with α-MEM or 10-fold-concentrated nocardial culture filtrate (n=4 per group) and activity was measured. The graphs illustrate proteasome activity relative to the respective negative control. Asterisks (*) note significant differences from the control (p<0.01).
Fig. 8.
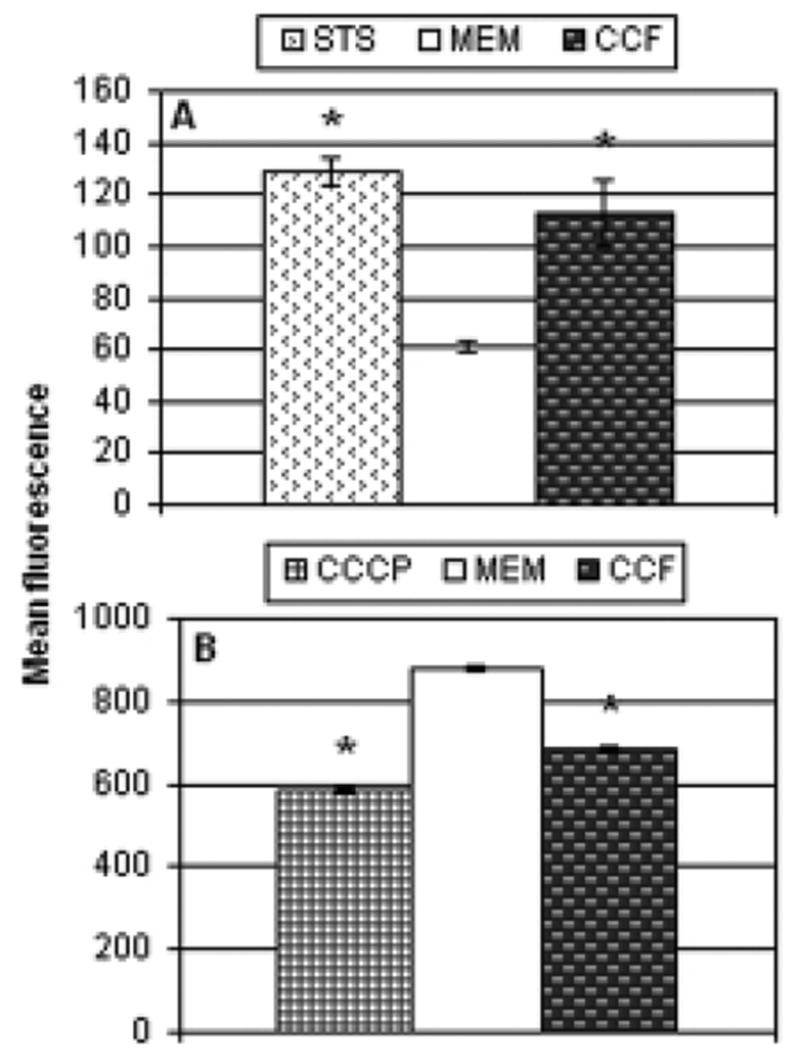
Apoptosis induced by concentrated culture filtrate. HeLa cells were incubated for 5 hours with 1:10 diluted concentrated culture filtrate. Following incubation, caspase activity (panel A) and mitochondrial depolarization (panel B) were determined as described in the materials and methods and in the legends of Figs. 5 and 7. The bars indicate the mean fluorescence of the cells in each treatment (n 3 per group), as determined by flow cytometry, with asterisks labeling those that were significantly different from the α-MEM control (p<0.01).
3.7 Inhibition of proteasome activity
Interference with the normal function of the proteasome can be detrimental to the cell and can lead to its death. We investigated the ability of N. asteroides GUH-2 to inhibit proteasome activity of HeLa cells (Fig. 9A) and PC12 cells (Fig. 9B). Epoxomicin, the positive control, inhibited all three enzymatic activities (p<0.01) in both cell lines. All activities were also significantly affected in PC12 cells exposed to nocardiae (47%, 69%, and 67% of chymotryptic, tryptic, and PGPH activities of BHI control, respectively). However, incubation of HeLa cells with live N. asteroides GUH-2 only significantly affected the chymotryptic (73% control) and PGPH-like (61% control) activities. The effect on the tryptic activity (89% control) was not significant.
To illustrate a specific effect on the proteasome, and not other proteases, experiments were performed using purified rabbit 20S proteasome incubated with concentrated nocardial culture filtrate (Fig. 9C). Exposure of purified proteasome to epoxomicin inhibited all three activities (data not shown). As was seen with HeLa cell lysates, exposure of proteasome to concentrated filtrate significantly reduced the activity of the chymotryptic (43% control) and PGPH activity (50% control), but not the tryptic activity (100% control).
4. Discussion
In the present study, the ability of N. asteroides GUH-2 to cause apoptotic death in epithelial cells was investigated. The results corroborate those in a previous report in which we described the effect of nocardial infection on the PC12 cell line and in the brains of mice [43]. The current study also expanded research on the pathways by which apoptosis is induced by nocardiae and added to our understanding of the bacterial components necessary for the induction of programmed cell death.
DNA fragmentation was observed in HeLa cells incubated with live N. asteroides (Figs. 1, 2, and 3), confirming our previous report, and suggesting that induced cell death is not limited to a particular cell type or organ. In addition, the experiments using the fluorescent pan-caspase inhibitor revealed that exposure to live exponential phase bacteria caused caspase activation (Fig. 5). The involvement of caspases is common to most described pathways of apoptosis [12, 48], including those triggered by other bacterial pathogens [25, 37, 38]. Further probing into downstream events in the pathway indicated that caspase-3, an executor caspase, was specifically activated (Fig. 6).
This study also revealed some specific participants in the induction of cell death. Mitochondrial disruption was observed in cells incubated with live GUH-2 (Fig. 7). Similar effects on these organelles have been noted in other reports of bacteria-induced apoptosis [22, 24, 38, 46]. Another potential mechanism for the induction of host cell death is proteasome inhibition. Inhibition of this protein complex was observed in cell lysates and with purified proteasome (Fig. 9). We believe this is the first report indicating that a species of Nocardia can induce proteasome inhibition.
Bacterial induced alteration of the apoptotic cascade has been reported for a growing number of different bacterial species [34], and the perspective of both the host cell and the bacterium must be considered to understand these interactions. Host cells, when challenged with intracellular bacteria, may undergo apoptosis as a means of expelling the pathogens, and exposing them to the components of the immune system. Thus, apoptosis can be a defense mechanism evolved in response to intracellular attack. Obligate intracellular pathogens such as Rickettsia or Chlamydia act to suppress the apoptotic cascade, thus prolonging their survival [5, 10]. Additionally, some reports on infection with different species of the facultative intracellular pathogen Mycobacterium have indicated that strains that are better able to prevent host cell apoptosis are more virulent, perhaps by enabling the bacteria to conceal themselves from the immune system [11].
From the bacterium’s perspective, the eukaryotic host is a dangerous place, containing a variety of cell types seeking its elimination. Many reports of bacterial induced cell death have focused on the infection and destruction of cells of the immune system [19, 34, 37, 38]. Such a response could lead to localized immune suppression at the site of infection. Apoptosis induced in other cell types, including epithelial and neuronal cells, has also been reported [1, 31, 34, 43, 46]. By triggering immunologically silent apoptotic cell death, rather than necrosis, inflammation and an influx of immune cells can be avoided [29].
In our initial experiments, only live bacteria had a significant effect on HeLa cells, compared with heat-killed N. asteroides or unconcentrated culture filtrate. Such results suggested a requirement for active bacterial processes in the induction of host cell death. Two possibilities appear probable. First, bacterial invasion of the host cell may be necessary. Second, apoptosis could be due to a localized concentration of some secreted product.
Although both killed and live bacteria were able to attach to cells (Fig. 4), only live N. asteroides were seen to invade HeLa cells. Similar effects were previously reported for endothelial cells in the murine brain [4]. This could account for the differing effects. In numerous repetitions of the assays, the effect of killed bacteria was always greater than that of unconcentrated filtrate, though it was not statistically different from the negative control. This observation could indicate that a few killed cells entered the HeLa hosts or that there was a slight killing effect due solely to the attachment of the bacteria. Another possible reason for seeing only insignificant increases in apoptosis in response to heat-killed bacteria could be differences in the multiplicity of infection compared to cells treated with live nocardiae. Although the initial doses of live and heat-killed bacteria were equivalent, growth of bacteria during the 5-hour exposure period could have altered the ratio. However, experiments designed to monitor nocardial growth indicated that there was almost no increase in nocardial numbers within this time (data not shown), probably because of a lag in bacterial replication as they adapted from the rich BHI broth in which they were cultured to the defined MEM tissue culture medium.
Regardless of whether or not invasion is necessary, bacteria may induce apoptosis by production of some toxic substance. Unconcentrated culture filtrate did not have an effect in these experiments, but this could have been because the low amounts of any reactive substances may not have reached the levels achievable by live bacteria actively secreting a toxic product at the site of infection. Such an effect due to secreted products could also account for the lack of apoptotic death induced by heat-killed bacteria, which retain the ability to bind to HeLa cells (Fig. 4C), but are metabolically inactive.
Since previous experiments using similar nocardial culture filtrates indicated that they did possess cytotoxic activity [6, 30], we sought to replicate this effect. In the earlier research, the host cells were exposed to undiluted culture filtrate. Here we treated cells with 10-fold concentrated culture filtrate diluted back to 1X. In addition, as one prior study [30] had suggested that the apoptosis-inducing activity was a small molecule that resided within the sub-3-kDa fraction of the culture filtrate we utilized similar culture filtrate from which all components larger than 8-kDa had been removed. Our experiments indicated that this concentrated culture filtrate did trigger caspase activation (Fig. 8A) and mitochondrial depolarization (Fig. 8B), indicating that neither bacterial invasion nor adherence were necessary for nocardial induced cell death.
A potential mechanism for the induction of apoptosis was also identified. Cultured cells exposed to live nocardiae exhibited signs of proteasome inhibition (Fig. 8). These observations were further confirmed using purified proteasome exposed to concentrated nocardial culture filtrate. Although it cannot be ruled out that the apoptosis-inducing factor is distinct from the proteasome inhibitor, recent studies have shown that natural proteasome inhibitors have similar capacities to induce apoptosis in vitro and in vivo [32, 39]. These compounds, small molecules with a peptide structure [21, 27, 42], which are produced and secreted by actinomycete species closely related to Nocardia [18, 20, 26], interfere with normal protein turnover in cells and can cause programmed cell death [47]. This proapoptotic activity may be due to extended longevity of caspases or modulation of expression of antiapoptotic Bcl-2, a regulator of the intrinsic apoptotic pathway.
The proteasome has three major activities, which are named for their similarity to those of other cellular proteases [40]. The natural proteasome inhibitors produced by actinomycetes do not affect these other enzymes and exhibit high specificity towards the proteasome [35], indicating that although the activities of the proteasome are similar to those other enzymes, the structures are not. In the experiments with HeLa cell lysates and with purified proteasome, it was noted that the trypsin-like activity of the proteasome was not inhibited. The natural proteasome inhibitors TMC-86A, TMC-86B, and TMC-96, isolated from Streptomyces sp. and Saccharothrix sp., similarly showed superior inhibition of the chymotryptic and PGPH activities [26]. Our observations suggest that the apparent proteasome inhibitor made by N. asteroides GUH-2 may be similar to those previously described.
The observations reported here appear to link some earlier independent studies. Infection of animals with members of the bacterial order Actinomycetales had been found to induce a Parkinsonian syndrome [13, 28]. Separately, McNaught et al. found that systemic administration of proteasome inhibitors produced by these bacteria to rats could cause Parkinsonism [32]. In their report, they speculated that an environmental source of these toxins could be soil bacteria. Our finding that N. asteroides GUH-2, a known parkinsinogenic strain of bacteria, is capable of causing both apoptosis and proteasome inhibition adds strength to the argument that bacteria may be involved in some cases of human Parkinson’s disease.
Although the ability of this bacterium to induce apoptosis may act as a virulence factor, contributing to its ability to cause illness, other pathogenic mechanisms also likely exist. N. asteroides strain 19247, the avirulent type strain of the species, was also tested using the TUNEL assay. There was no difference from virulent strain GUH-2—both significantly increased the level of DNA fragmentation compared to the BHI control (data not shown). This finding suggests that other as yet unidentified virulence factors likely contribute to the induction of disease by Nocardia. This report supports the hypothesis that nocardial induced host cell death occurs via an apoptotic mechanism and is not specific to any particular cell type. Future planned studies will investigate the involvement of specific caspases and particular pathways of apoptosis and will explore the nature of the nocardial proteasome inhibitor.
Acknowledgments
The authors wish to thank Terri Ellis and Susanne Berglund for their critical reading of this manuscript. This work was funded in part by Public Health Service Grants R01-AI42144 from the National Institute for Allergy and Infectious Diseases and R01-HL59821 from the National Heart, Lung, and Blood Institute. Some of the work was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR-12088-01 from the National Center for Research Resources, National Institutes of Health. Some of these data were presented previously at the 104th General Meeting of the American Society for Microbiology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abul-Milh M, Wu Y, Lau B, Lingwood CA, Foster DB. Induction of epithelial cell death including apoptosis by enteropathogenic Escherichia coli expressing bundle-forming pili. Infect Immun. 2001;69:7356–7364. doi: 10.1128/IAI.69.12.7356-7364.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bandopadhyay R, Kingsbury AE, Muqit MM, Harvey K, Reid AR, Kilford L, Engelender S, Schlossmacher MG, Wood NW, Latchman DS, Harvey RJ, Lees AJ. Synphilin-1 and parkin show overlapping expression patterns in human brain and form aggresomes in response to proteasomal inhibition. Neurobiol Dis. 2005;20:401–411. doi: 10.1016/j.nbd.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 3.Beaman BL, Beaman L. Nocardia species: host-parasite relationships. Clin Microbiol Rev. 1994;7:213–264. doi: 10.1128/cmr.7.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaman BL, Ogata SA. Ultrastructural analysis of attachment to and penetration of capillaries in the murine pons, midbrain, thalamus, and hypothalamus by Nocardia asteroides. Infect Immun. 1993;61:955–965. doi: 10.1128/iai.61.3.955-965.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byrne GI, Ojcius DM. Chlamydia and apoptosis: life and death decisions of an intracellular pathogen. Nat Rev Microbiol. 2004;2:802–808. doi: 10.1038/nrmicro1007. [DOI] [PubMed] [Google Scholar]
- 6.Camp DM, Loeffler DA, Razoky BA, Tam S, Beaman BL, LeWitt PA. Nocardia asteroides culture filtrates cause dopamine depletion and cytotoxicity in PC12 cells. Neurochem Res. 2003;28:1359–1367. doi: 10.1023/a:1024944431725. [DOI] [PubMed] [Google Scholar]
- 7.Carreras I, Garrett-Young R, Ullman MD, Eisenhauer PB, Fine RE, Wells JM, Conn KJ. Upregulation of clusterin/apolipoprotein J in lactacystin-treated SH-SY5Y cells. J Neurosci Res. 2005;79:495–502. doi: 10.1002/jnr.20374. [DOI] [PubMed] [Google Scholar]
- 8.Chapman G, Beaman BL, Loeffler DA, Camp DM, Domino EF, Dickson DW, Ellis WG, Chen I, Bachus SE, LeWitt PA. In situ hybridization for detection of nocardial 16S rRNA: reactivity within intracellular inclusions in experimentally infected cynomolgus monkeys--and in Lewy body-containing human brain specimens. Exp Neurol. 2003;184:715–725. doi: 10.1016/S0014-4886(03)00337-6. [DOI] [PubMed] [Google Scholar]
- 9.Chen W, Lee J, Cho SY, Fine HA. Proteasome-mediated destruction of the cyclin a/cyclin-dependent kinase 2 complex suppresses tumor cell growth in vitro and in vivo. Cancer Res. 2004;64:3949–3957. doi: 10.1158/0008-5472.CAN-03-3906. [DOI] [PubMed] [Google Scholar]
- 10.Clifton DR, Goss RA, Sahni SK, van Antwerp D, Baggs RB, Marder VJ, Silverman DJ, Sporn LA. NF-kappa B-dependent inhibition of apoptosis is essential for host cell survival during Rickettsia rickettsii infection. Proc Natl Acad Sci U S A. 1998;95:4646–4651. doi: 10.1073/pnas.95.8.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danelishvili L, McGarvey J, Li YJ, Bermudez LE. Mycobacterium tuberculosis infection causes different levels of apoptosis and necrosis in human macrophages and alveolar epithelial cells. Cell Microbiol. 2003;5:649–660. doi: 10.1046/j.1462-5822.2003.00312.x. [DOI] [PubMed] [Google Scholar]
- 12.Denecker G, Vercammen D, Declercq W, Vandenabeele P. Apoptotic and necrotic cell death induced by death domain receptors. Cell Mol Life Sci. 2001;58:356–370. doi: 10.1007/PL00000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaz-Corrales FJ, Colasante C, Contreras Q, Puig M, Serrano JA, Hernandez L, Beaman BL. Nocardia otitidiscaviarum (GAM-5) induces parkinsonian-like alterations in mouse. Braz J Med Biol Res. 2004;37:539–548. doi: 10.1590/s0100-879x2004000400011. [DOI] [PubMed] [Google Scholar]
- 14.Ekert PG, Vaux DL. Apoptosis and the immune system. Br Med Bull. 1997;53:591–603. doi: 10.1093/oxfordjournals.bmb.a011632. [DOI] [PubMed] [Google Scholar]
- 15.Elliott PJ, Soucy TA, Pien CS, Adams J, Lightcap ES. Assays for proteasome inhibition. Methods Mol Med. 2003;85:163–172. doi: 10.1385/1-59259-380-1:163. [DOI] [PubMed] [Google Scholar]
- 16.Fadeel B. Programmed cell clearance. Cell Mol Life Sci. 2003;60:2575–2585. doi: 10.1007/s00018-003-3145-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fearnley J, Lees A. In: Neurodegenerative Diseases. Calne DB, editor. Saunders; Philadelphia: 1994. pp. 545–554. [Google Scholar]
- 18.Feling RH, Buchanan GO, Mincer TJ, Kauffman CA, Jensen PR, Fenical W. Salinosporamide A: a highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus Salinospora. Angew Chem Int Ed Engl. 2003;42:355–357. doi: 10.1002/anie.200390115. [DOI] [PubMed] [Google Scholar]
- 19.Fettucciari K, Rosati E, Scaringi L, Cornacchione P, Migliorati G, Sabatini R, Fetriconi I, Rossi R, Marconi P. Group B Streptococcus induces apoptosis in macrophages. J Immunol. 2000;165:3923–3933. doi: 10.4049/jimmunol.165.7.3923. [DOI] [PubMed] [Google Scholar]
- 20.Figueiredo-Pereira ME, Chen WE, Li J, Johdo O. The antitumor drug aclacinomycin A, which inhibits the degradation of ubiquitinated proteins, shows selectivity for the chymotrypsin-like activity of the bovine pituitary 20 S proteasome. J Biol Chem. 1996;271:16455–16459. doi: 10.1074/jbc.271.28.16455. [DOI] [PubMed] [Google Scholar]
- 21.Hanada M, Sugawara K, Kaneta K, Toda S, Nishiyama Y, Tomita K, Yamamoto H, Konishi M, Oki T. Epoxomicin, a new antitumor agent of microbial origin. J Antibiot (Tokyo) 1992;45:1746–1752. doi: 10.7164/antibiotics.45.1746. [DOI] [PubMed] [Google Scholar]
- 22.He D, Hagen SJ, Pothoulakis C, Chen M, Medina ND, Warny M, LaMont JT. Clostridium difficile toxin A causes early damage to mitochondria in cultured cells. Gastroenterology. 2000;119:139–150. doi: 10.1053/gast.2000.8526. [DOI] [PubMed] [Google Scholar]
- 23.Hipfner DR, Cohen SM. Connecting proliferation and apoptosis in development and disease. Nat Rev Mol Cell Biol. 2004;5:805–815. doi: 10.1038/nrm1491. [DOI] [PubMed] [Google Scholar]
- 24.Jan G, Belzacq AS, Haouzi D, Rouault A, Metivier D, Kroemer G, Brenner C. Propionibacteria induce apoptosis of colorectal carcinoma cells via short-chain fatty acids acting on mitochondria. Cell Death Differ. 2002;9:179–188. doi: 10.1038/sj.cdd.4400935. [DOI] [PubMed] [Google Scholar]
- 25.Jarvelainen HA, Galmiche A, Zychlinsky A. Caspase-1 activation by Salmonella. Trends Cell Biol. 2003;13:204–209. doi: 10.1016/s0962-8924(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 26.Koguchi Y, Kohno J, Suzuki S, Nishio M, Takahashi K, Ohnuki T, Komatsubara S. TMC-86A, B and TMC-96, new proteasome inhibitors from Streptomyces sp. TC 1084 and Saccharothrix sp. TC 1094. I. Taxonomy, fermentation, isolation, and biological activities. J Antibiot (Tokyo) 1999;52:1069–1076. doi: 10.7164/antibiotics.52.1069. [DOI] [PubMed] [Google Scholar]
- 27.Koguchi Y, Kohno J, Suzuki S, Nishio M, Takahashi K, Ohnuki T, Komatsubara S. TMC-86A, B and TMC-96, new proteasome inhibitors from Streptomyces sp. TC 1084 and Saccharothrix sp. TC 1094. II. Physicochemical properties and structure determination. J Antibiot (Tokyo) 2000;53:63–65. doi: 10.7164/antibiotics.53.63. [DOI] [PubMed] [Google Scholar]
- 28.Kohbata S, Beaman BL. L-dopa-responsive movement disorder caused by Nocardia asteroides localized in the brains of mice. Infect Immun. 1991;59:181–191. doi: 10.1128/iai.59.1.181-191.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurosaka K, Takahashi M, Watanabe N, Kobayashi Y. Silent cleanup of very early apoptotic cells by macrophages. J Immunol. 2003;171:4672–4679. doi: 10.4049/jimmunol.171.9.4672. [DOI] [PubMed] [Google Scholar]
- 30.Loeffler DA, Camp DM, Qu S, Beaman BL, LeWitt PA. Characterization of dopamine-depleting activity of Nocardia asteroides strain GUH-2 culture filtrate on PC12 cells. Microb Pathog. 2004;37:73–85. doi: 10.1016/j.micpath.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Massari P, King CA, Ho AY, Wetzler LM. Neisserial PorB is translocated to the mitochondria of HeLa cells infected with Neisseria meningitidis and protects cells from apoptosis. Cell Microbiol. 2003;5:99–109. doi: 10.1046/j.1462-5822.2003.00257.x. [DOI] [PubMed] [Google Scholar]
- 32.McNaught KS, Perl DP, Brownell AL, Olanow CW. Systemic exposure to proteasome inhibitors causes a progressive model of Parkinson's disease. Ann Neurol. 2004;56:149–162. doi: 10.1002/ana.20186. [DOI] [PubMed] [Google Scholar]
- 33.Monti D, Grassilli E, Troiano L, Cossarizza A, Salvioli S, Barbieri D, Agnesini C, Bettuzzi S, Ingletti MC, Corti A, et al. Senescence, immortalization, and apoptosis. An intriguing relationship. Ann N Y Acad Sci. 1992;673:70–82. doi: 10.1111/j.1749-6632.1992.tb27438.x. [DOI] [PubMed] [Google Scholar]
- 34.Moss JE, Aliprantis AO, Zychlinsky A. The regulation of apoptosis by microbial pathogens. Int Rev Cytol. 1999;187:203–259. doi: 10.1016/s0074-7696(08)62419-5. [DOI] [PubMed] [Google Scholar]
- 35.Mykles DL. Proteinase families and their inhibitors. Methods Cell Biol. 2001;66:247–287. doi: 10.1016/s0091-679x(01)66012-6. [DOI] [PubMed] [Google Scholar]
- 36.Noda K, Kitami T, Gai WP, Chegini F, Jensen PH, Fujimura T, Murayama K, Tanaka K, Mizuno Y, Hattori N. Phosphorylated IkappaBalpha is a component of Lewy body of Parkinson's disease. Biochem Biophys Res Commun. 2005;331:309–317. doi: 10.1016/j.bbrc.2005.03.167. [DOI] [PubMed] [Google Scholar]
- 37.Perskvist N, Long M, Stendahl O, Zheng L. Mycobacterium tuberculosis promotes apoptosis in human neutrophils by activating caspase-3 and altering expression of Bax/Bcl-xL via an oxygen-dependent pathway. J Immunol. 2002;168:6358–6365. doi: 10.4049/jimmunol.168.12.6358. [DOI] [PubMed] [Google Scholar]
- 38.Popov SG, Villasmil R, Bernardi J, Grene E, Cardwell J, Wu A, Alibek D, Bailey C, Alibek K. Lethal toxin of Bacillus anthracis causes apoptosis of macrophages. Biochem Biophys Res Commun. 2002;293:349–355. doi: 10.1016/S0006-291X(02)00227-9. [DOI] [PubMed] [Google Scholar]
- 39.Rideout HJ, Dietrich P, Wang Q, Dauer WT, Stefanis L. alpha-synuclein is required for the fibrillar nature of ubiquitinated inclusions induced by proteasomal inhibition in primary neurons. J Biol Chem. 2004;279:46915–46920. doi: 10.1074/jbc.M405146200. [DOI] [PubMed] [Google Scholar]
- 40.Rivett AJ. The multicatalytic proteinase. Multiple proteolytic activities. J Biol Chem. 1989;264:12215–12219. [PubMed] [Google Scholar]
- 41.Rojas M, Barrera LF, Puzo G, Garcia LF. Differential induction of apoptosis by virulent Mycobacterium tuberculosis in resistant and susceptible murine macrophages: role of nitric oxide and mycobacterial products. J Immunol. 1997;159:1352–1361. [PubMed] [Google Scholar]
- 42.Sugawara K, Hatori M, Nishiyama Y, Tomita K, Kamei H, Konishi M, Oki T. Eponemycin, a new antibiotic active against B16 melanoma. I. Production, isolation, structure and biological activity. J Antibiot (Tokyo) 1990;43:8–18. doi: 10.7164/antibiotics.43.8. [DOI] [PubMed] [Google Scholar]
- 43.Tam S, Barry DP, Beaman L, Beaman BL. Neuroinvasive Nocardia asteroides GUH-2 induces apoptosis in the substantia nigra in vivo and dopaminergic cells in vitro. Exp Neurol. 2002;177:453–460. doi: 10.1006/exnr.2002.8012. [DOI] [PubMed] [Google Scholar]
- 44.Voges D, Zwickl P, Baumeister W. The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu Rev Biochem. 1999;68:1015–1068. doi: 10.1146/annurev.biochem.68.1.1015. [DOI] [PubMed] [Google Scholar]
- 45.Weng C, Li Y, Xu D, Shi Y, Tang H. Specific cleavage of Mcl-1 by caspase-3 in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in Jurkat leukemia T cells. J Biol Chem. 2005;280:10491–10500. doi: 10.1074/jbc.M412819200. [DOI] [PubMed] [Google Scholar]
- 46.Willhite DC, Blanke SR. Helicobacter pylori vacuolating cytotoxin enters cells, localizes to the mitochondria, and induces mitochondrial membrane permeability changes correlated to toxin channel activity. Cell Microbiol. 2004;6:143–154. doi: 10.1046/j.1462-5822.2003.00347.x. [DOI] [PubMed] [Google Scholar]
- 47.Wojcik C. Proteasomes in apoptosis: villains or guardians? Cell Mol Life Sci. 1999;56:908–917. doi: 10.1007/s000180050483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolf BB, Green DR. Suicidal tendencies: apoptotic cell death by caspase family proteinases. J Biol Chem. 1999;274:20049–20052. doi: 10.1074/jbc.274.29.20049. [DOI] [PubMed] [Google Scholar]
- 49.Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]


