Abstract
The effects of temperature on strains of Escherichia coli which overproduce and excrete either beta-lactamase or human epidermal growth factor were investigated. E. coli RB791 cells containing plasmid pKN which has the tac promoter upstream of the gene for beta-lactamase were grown and induced with isopropyl-beta-D-thiogalactopyranoside in batch culture at 37, 30, 25, and 20 degrees C. The lower temperature greatly reduced the formation of periplasmic beta-lactamase inclusion bodies, increased significantly the total amount of beta-lactamase activity, and increased the purity of extracellular beta-lactamase from approximately 45 to 90%. Chemostat operation at 37 and 30 degrees C was difficult due to poor cell reproduction and beta-lactamase production. However, at 20 degrees C, continuous production and excretion of beta-lactamase were obtained for greater than 450 h (29 generations). When the same strain carried plasmid pCU encoding human epidermal growth factor, significant cell lysis was observed after induction at 31 and 37 degrees C, whereas little cell lysis was observed at 21 and 25 degrees C. Both total soluble and total human epidermal growth factor increased with decreasing temperature. These results indicate that some of the problems of instability of strains producing high levels of plasmid-encoded proteins can be mitigated by growth at lower temperatures. Further, lower temperatures can increase for at least some secreted proteins both total plasmid-encoded protein formed and the fraction that is soluble.
Full text
PDF
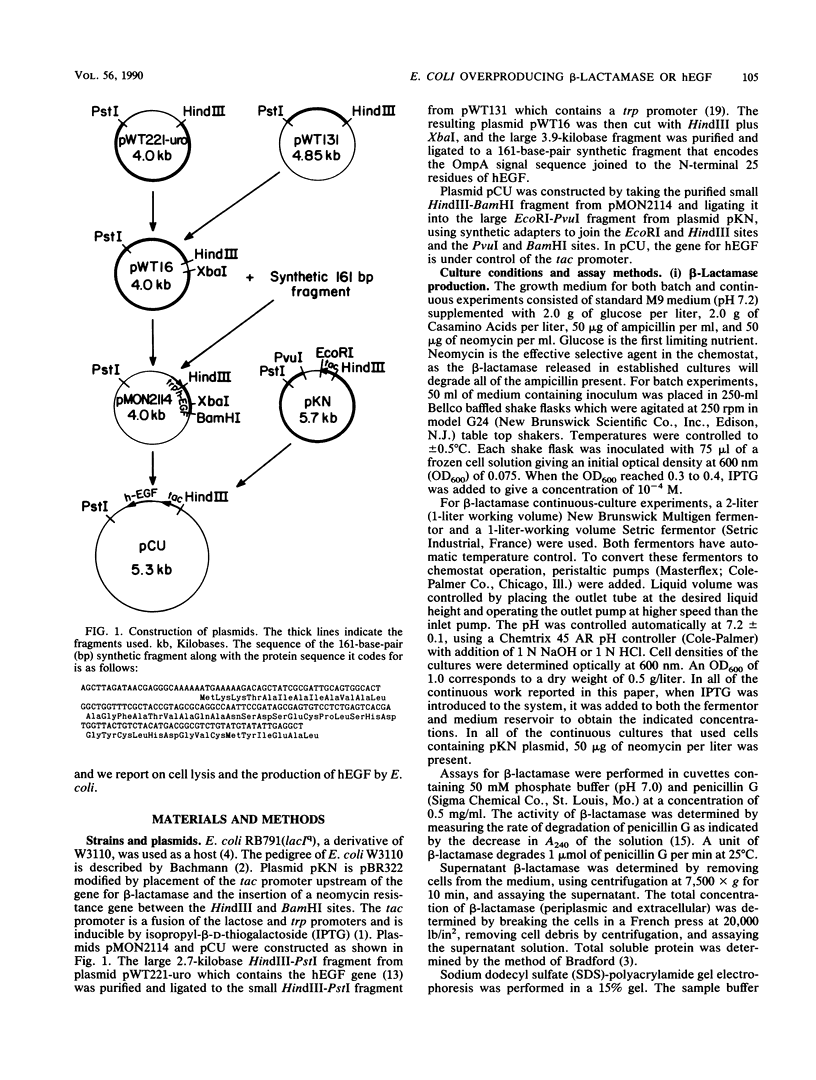






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann E., Brosius J., Ptashne M. Vectors bearing a hybrid trp-lac promoter useful for regulated expression of cloned genes in Escherichia coli. Gene. 1983 Nov;25(2-3):167–178. doi: 10.1016/0378-1119(83)90222-6. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brent R., Ptashne M. Mechanism of action of the lexA gene product. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4204–4208. doi: 10.1073/pnas.78.7.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikes J. D., Bassford P. J., Jr Export of unprocessed precursor maltose-binding protein to the periplasm of Escherichia coli cells. J Bacteriol. 1987 Jun;169(6):2352–2359. doi: 10.1128/jb.169.6.2352-2359.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou G., Telford J. N., Shuler M. L., Wilson D. B. Localization of inclusion bodies in Escherichia coli overproducing beta-lactamase or alkaline phosphatase. Appl Environ Microbiol. 1986 Nov;52(5):1157–1161. doi: 10.1128/aem.52.5.1157-1161.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. N., Schwartz M., Silhavy T. J. Sequence information within the lamB genes in required for proper routing of the bacteriophage lambda receptor protein to the outer membrane of Escherichia coli K-12. J Mol Biol. 1982 Mar 25;156(1):93–112. doi: 10.1016/0022-2836(82)90461-2. [DOI] [PubMed] [Google Scholar]
- Imanaka T., Aiba S. A perspective on the application of genetic engineering: stability of recombinant plasmid. Ann N Y Acad Sci. 1981;369:1–14. doi: 10.1111/j.1749-6632.1981.tb14172.x. [DOI] [PubMed] [Google Scholar]
- Samuni A. A direct spectrophotometric assay and determination of Michaelis constants for the beta-lactamase reaction. Anal Biochem. 1975 Jan;63(1):17–26. doi: 10.1016/0003-2697(75)90185-2. [DOI] [PubMed] [Google Scholar]
- Sharma S. K., Evans D. B., Tomich C. S., Cornette J. C., Ulrich R. G. Folding and activation of recombinant human prorenin. Biotechnol Appl Biochem. 1987 Apr;9(2):181–193. [PubMed] [Google Scholar]
- Tacon W., Carey N., Emtage S. The construction and characterisation of plasmid vectors suitable for the expression of all DNA phases under the control of the E. coli tryptophan promoter. Mol Gen Genet. 1980 Feb;177(3):427–438. doi: 10.1007/BF00271481. [DOI] [PubMed] [Google Scholar]
- Tommassen J., Leunissen J., van Damme-Jongsten M., Overduin P. Failure of E. coli K-12 to transport PhoE-LacZ hybrid proteins out of the cytoplasm. EMBO J. 1985 Apr;4(4):1041–1047. doi: 10.1002/j.1460-2075.1985.tb03736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji T., Nakagawa R., Sugimoto N., Fukuhara K. Characterization of disulfide bonds in recombinant proteins: reduced human interleukin 2 in inclusion bodies and its oxidative refolding. Biochemistry. 1987 Jun 2;26(11):3129–3134. doi: 10.1021/bi00385a028. [DOI] [PubMed] [Google Scholar]
- Weir M. P., Sparks J. Purification and renaturation of recombinant human interleukin-2. Biochem J. 1987 Jul 1;245(1):85–91. doi: 10.1042/bj2450085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. C., Van Frank R. M., Muth W. L., Burnett J. P. Cytoplasmic inclusion bodies in Escherichia coli producing biosynthetic human insulin proteins. Science. 1982 Feb 5;215(4533):687–689. doi: 10.1126/science.7036343. [DOI] [PubMed] [Google Scholar]



