Abstract
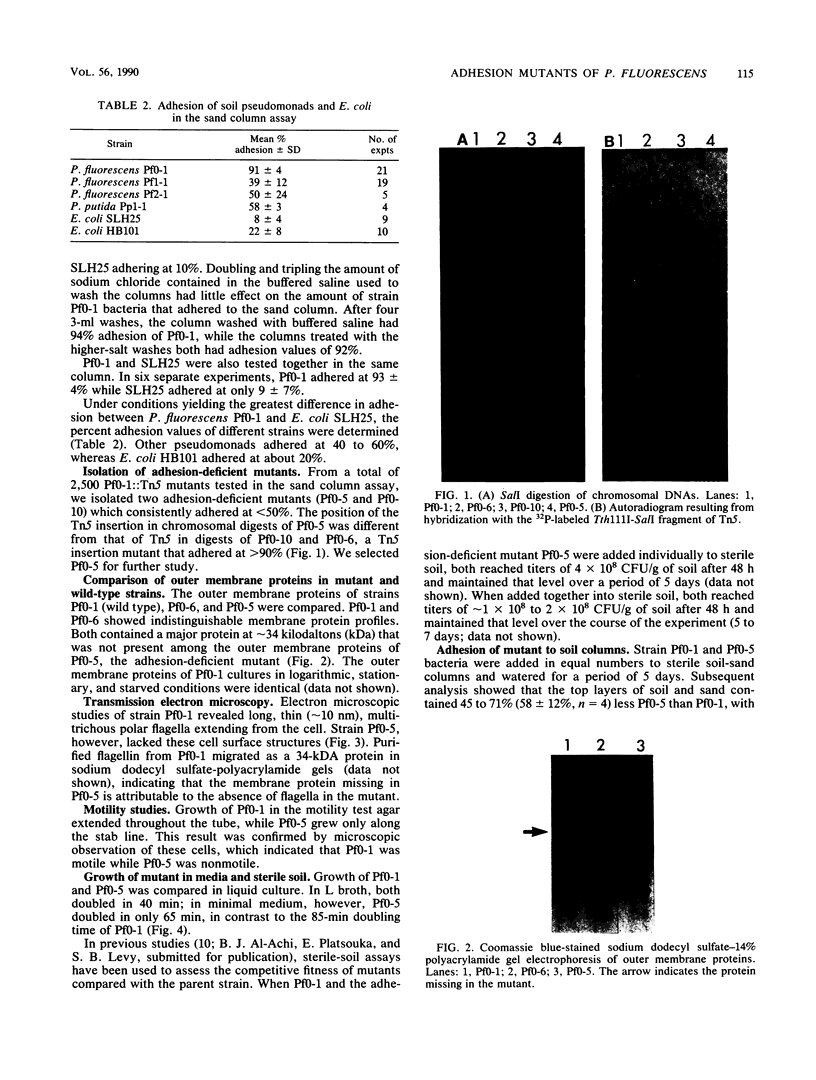
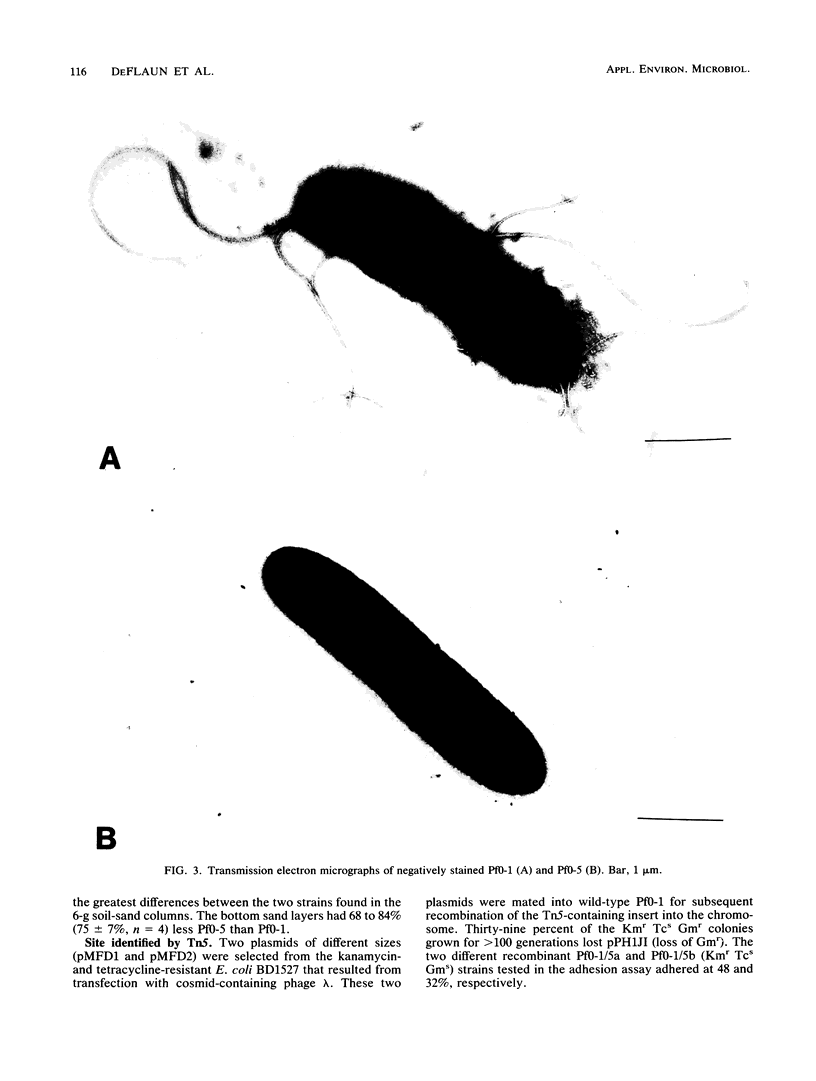
A sand column adhesion assay was developed which distinguishes the adhesion abilities of a number of pseudomonads isolated from fine sandy loam. Pseudomonas fluorescens Pf0-1 which adhered at >90% of the total cells added was subjected to transposon Tn5 insertion mutagenesis. From 2,500 Pf0-1::Tn5 mutants examined in the sand column assay, two adhesion-deficient Pf0-1 mutants showing <50% attachment were isolated. Marker exchange analysis of one of these mutants, Pf0-5, confirmed that the decreased adhesion was linked to the Tn5 insertion in the chromosome. The growth rate of Pf0-5 in enriched media and sterile soil was similar to that of the wild type; in minimal medium, however, Pf0-5 grew faster. In a soil column assay, less Pf0-5 than wild-type bacteria were recovered, suggesting a decreased ability to persist in soil. A 34-kilodalton major outer membrane protein present in the wild type was missing in Pf0-5. Transmission electron microscopy of the cell surface revealed that the wild-type possessed polar flagella which were absent in the mutant.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attridge S. R., Rowley D. The role of the flagellum in the adherence of Vibrio cholerae. J Infect Dis. 1983 May;147(5):864–872. doi: 10.1093/infdis/147.5.864. [DOI] [PubMed] [Google Scholar]
- Beji A., Izard D., Gavini F., Leclerc H., Leseine-Delstanche M., Krembel J. A rapid chemical procedure for isolation and purification of chromosomal DNA from gram-negative bacilli. Anal Biochem. 1987 Apr;162(1):18–23. doi: 10.1016/0003-2697(87)90005-4. [DOI] [PubMed] [Google Scholar]
- Belas M. R., Colwell R. R. Adsorption kinetics of laterally and polarly flagellated Vibrio. J Bacteriol. 1982 Sep;151(3):1568–1580. doi: 10.1128/jb.151.3.1568-1580.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Chow W. Y., Berg D. E. Tn5tac1, a derivative of transposon Tn5 that generates conditional mutations. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6468–6472. doi: 10.1073/pnas.85.17.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. P., McMurry L. M., Levy S. B. marA locus causes decreased expression of OmpF porin in multiple-antibiotic-resistant (Mar) mutants of Escherichia coli. J Bacteriol. 1988 Dec;170(12):5416–5422. doi: 10.1128/jb.170.12.5416-5422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compeau G., Al-Achi B. J., Platsouka E., Levy S. B. Survival of rifampin-resistant mutants of Pseudomonas fluorescens and Pseudomonas putida in soil systems. Appl Environ Microbiol. 1988 Oct;54(10):2432–2438. doi: 10.1128/aem.54.10.2432-2438.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Weger L. A., van der Vlugt C. I., Wijfjes A. H., Bakker P. A., Schippers B., Lugtenberg B. Flagella of a plant-growth-stimulating Pseudomonas fluorescens strain are required for colonization of potato roots. J Bacteriol. 1987 Jun;169(6):2769–2773. doi: 10.1128/jb.169.6.2769-2773.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C. J., Staneloni R. J., Rubin R. A., Nester E. W. Identification and genetic analysis of an Agrobacterium tumefaciens chromosomal virulence region. J Bacteriol. 1985 Mar;161(3):850–860. doi: 10.1128/jb.161.3.850-860.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A. M., Long S. R., Brown S. E., Buikema W. J., Ausubel F. M. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982 Jun;18(3):289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- Kieser T. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid. 1984 Jul;12(1):19–36. doi: 10.1016/0147-619x(84)90063-5. [DOI] [PubMed] [Google Scholar]
- Kjelleberg S., Humphrey B. A., Marshall K. C. Initial phases of starvation and activity of bacteria at surfaces. Appl Environ Microbiol. 1983 Nov;46(5):978–984. doi: 10.1128/aem.46.5.978-984.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall B., Flynn P., Kamely D., Levy S. B. Survival of Escherichia coli with and without ColE1::Tn5 after aerosol dispersal in a laboratory and a farm environment. Appl Environ Microbiol. 1988 Jul;54(7):1776–1783. doi: 10.1128/aem.54.7.1776-1783.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. H., Savage D. C. Purification and characterization of flagella from Roseburia cecicola, an obligately anaerobic bacterium. J Gen Microbiol. 1985 Aug;131(8):2075–2078. doi: 10.1099/00221287-131-8-2075. [DOI] [PubMed] [Google Scholar]
- Meselson M., Yuan R. DNA restriction enzyme from E. coli. Nature. 1968 Mar 23;217(5134):1110–1114. doi: 10.1038/2171110a0. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Uyen H. M., van der Mei H. C., Weerkamp A. H., Busscher H. J. Comparison between the adhesion to solid substrata of Streptococcus mitis and that of polystyrene particles. Appl Environ Microbiol. 1988 Mar;54(3):837–838. doi: 10.1128/aem.54.3.837-838.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]