Abstract
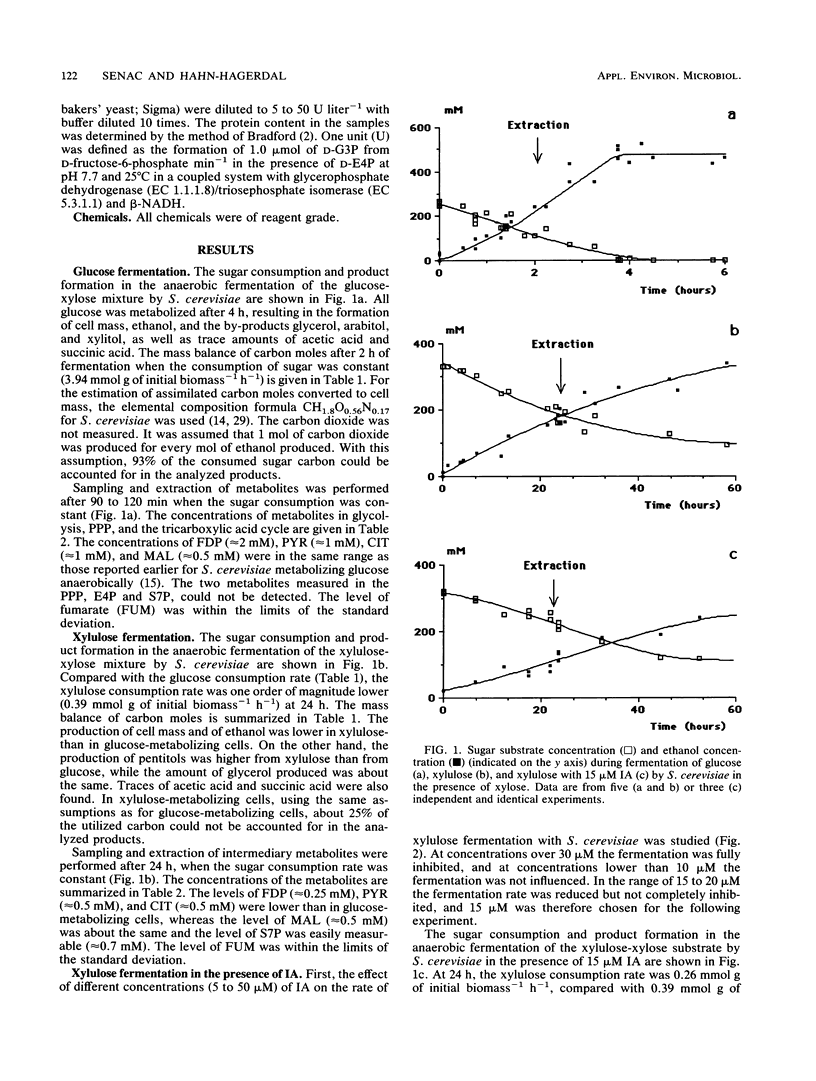
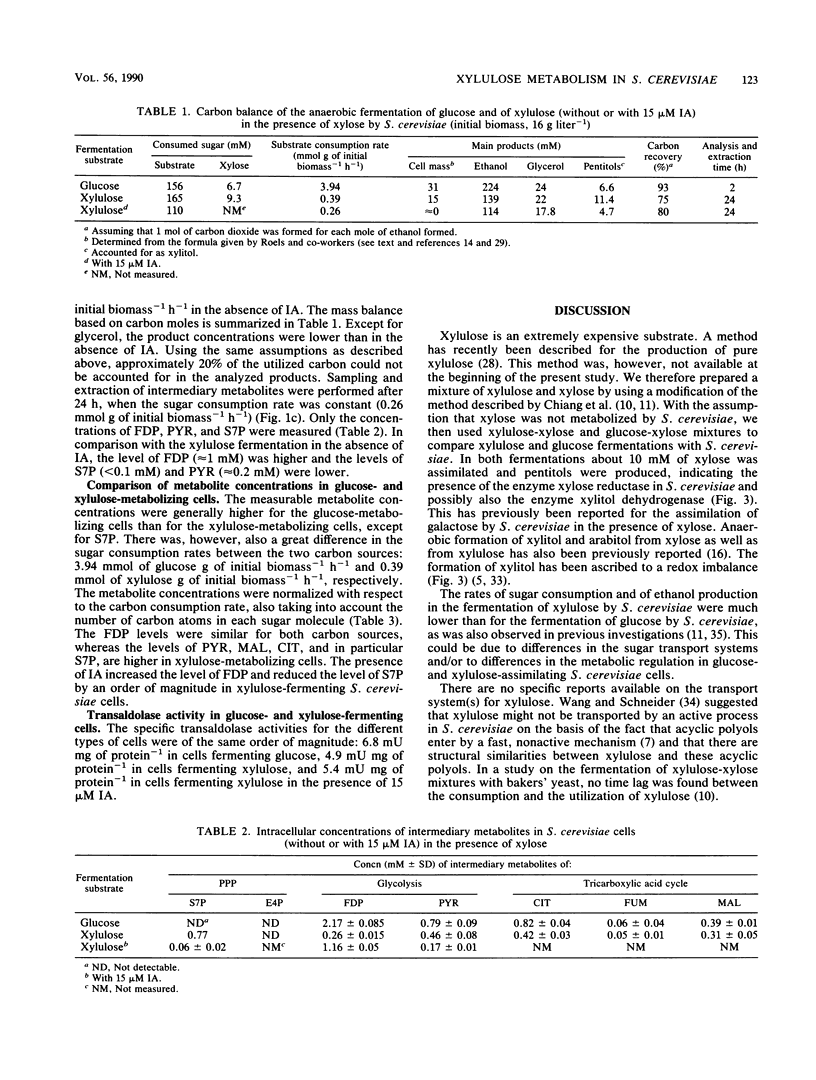
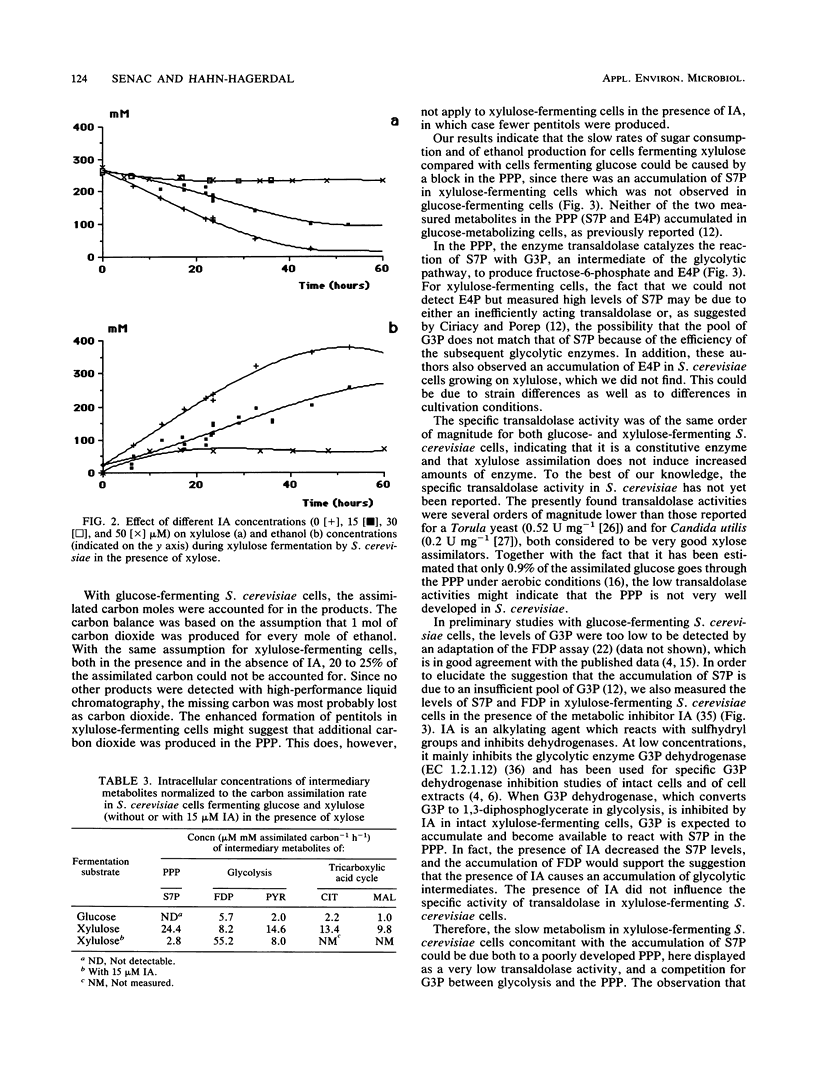
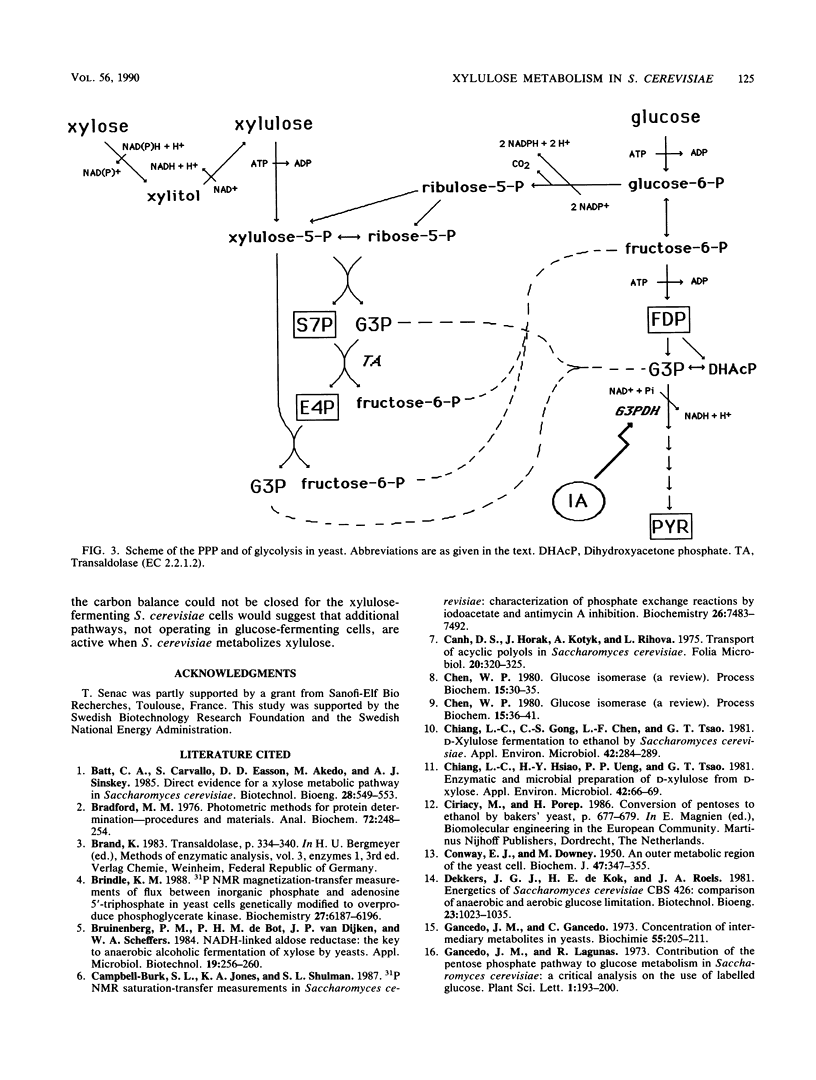
Glucose and xylulose fermentation and product formation by Saccharomyces cerevisiae were compared in batch culture under anaerobic conditions. In both cases the main product was ethanol, with glycerol, xylitol, and arabitol produced as by-products. During glucose and xylulose fermentation, 0.74 and 0.37 g of cell mass liter−1, respectively, were formed. In glucose-fermenting cells, the carbon balance could be closed, whereas in xylulose-fermenting cells, about 25% of the consumed sugar carbon could not be accounted for. The rate of sugar consumption was 3.94 mmol g of initial biomass−1 h−1 for glucose and 0.39 mmol g of initial biomass−1 h−1 for xylulose. Concentrations of the intermediary metabolites fructose-1,6-diphosphate (FDP), pyruvate (PYR), sedoheptulose 7-phosphate (S7P), erytrose 4-phosphate, citrate (CIT), fumarate, and malate were compared for both types of cells. Levels of FDP, PYR, and CIT were lower, and levels of S7P were higher in xylulose-fermenting cells. After normalization to the carbon consumption rate, the levels of FDP were approximately the same, whereas there was a significant accumulation of S7P, PYR, CIT, and malate, especially of S7P, in xylulose-fermenting cells compared with in glucose-fermenting cells. In the presence of 15 μM iodoacetate, an inhibitor of the enzyme glyceraldehyde-3-phosphate dehydrogenase (EC 1.2.1.12), FDP levels increased and S7P levels decreased in xylulose-assimilating cells compared with in the absence of the inhibitor, whereas fermentation was slightly slowed down. The specific activity of transaldolase (EC 2.2.1.2), the pentose phosphate pathway enzyme reacting with S7P and glyceraldehyde-3-phosphate, was essentially the same for both glucose- and xylulose-fermenting cells. It was, however, several orders of magnitude lower than that reported for a Torula yeast and Candida utilis. The presence of iodoacetate did not influence the activity of transaldolase in xylulose-fermenting cells. The results are discussed in terms of a competition between the pentose phosphate pathway and glycolysis for the common metabolite, glyceraldehyde-3-phosphate, which would explain the low rates of xylulose assimilation and ethanol production from xylulose by S. cerevisiae.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brindle K. M. 31P NMR magnetization-transfer measurements of flux between inorganic phosphate and adenosine 5'-triphosphate in yeast cells genetically modified to overproduce phosphoglycerate kinase. Biochemistry. 1988 Aug 9;27(16):6187–6196. doi: 10.1021/bi00416a054. [DOI] [PubMed] [Google Scholar]
- CONWAY E. J., DOWNEY M. An outer metabolic region of the yeast cell. Biochem J. 1950 Sep;47(3):347–355. doi: 10.1042/bj0470347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Burk S. L., Jones K. A., Shulman R. G. 31P NMR saturation-transfer measurements in Saccharomyces cerevisiae: characterization of phosphate exchange reactions by iodoacetate and antimycin A inhibition. Biochemistry. 1987 Nov 17;26(23):7483–7492. doi: 10.1021/bi00397a043. [DOI] [PubMed] [Google Scholar]
- Canh D. S., Horák J., Kotyk A., Ríhová L. Transport of acyclic polyols in Saccharomyces cerevisiae. Folia Microbiol (Praha) 1975;20(4):320–325. doi: 10.1007/BF02878113. [DOI] [PubMed] [Google Scholar]
- Chiang L. C., Gong C. S., Chen L. F., Tsao G. T. d-Xylulose Fermentation to Ethanol by Saccharomyces cerevisiae. Appl Environ Microbiol. 1981 Aug;42(2):284–289. doi: 10.1128/aem.42.2.284-289.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang L. C., Hsiao H. Y., Ueng P. P., Tsao G. T. Enzymatic and Microbial Preparation of d-Xylulose from d-Xylose. Appl Environ Microbiol. 1981 Jul;42(1):66–69. doi: 10.1128/aem.42.1.66-69.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancedo J. M., Gancedo C. Concentrations of intermediary metabolites in yeast. Biochimie. 1973;55(2):205–211. doi: 10.1016/s0300-9084(73)80393-1. [DOI] [PubMed] [Google Scholar]
- Lohmeier-Vogel E., Skoog K., Vogel H., Hahn-Hägerdal B. 31P nuclear magnetic resonance study of the effect of azide on xylose fermentation by Candida tropicalis. Appl Environ Microbiol. 1989 Aug;55(8):1974–1980. doi: 10.1128/aem.55.8.1974-1980.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PONTREMOLI S., BONSIGNORE A., GRAZI E., HORECKER B. L. A coupled reaction catalyzed by the enzymes transketolase and transaldolase. J Biol Chem. 1960 Jul;235:1881–1887. [PubMed] [Google Scholar]
- PONTREMOLI S., PRANDINI B. D., BONSIGNORE A., HORECKER B. L. The preparation of crystalline transaldolase from Candida utilis. Proc Natl Acad Sci U S A. 1961 Dec 15;47:1942–1949. doi: 10.1073/pnas.47.12.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöberg A., Hahn-Hägerdal B. beta-Glucose-1-Phosphate, a Possible Mediator for Polysaccharide Formation in Maltose-Assimilating Lactococcus lactis. Appl Environ Microbiol. 1989 Jun;55(6):1549–1554. doi: 10.1128/aem.55.6.1549-1554.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P. Y., Schneider H. Growth of yeasts on D-xylulose 1. Can J Microbiol. 1980 Sep;26(9):1165–1168. doi: 10.1139/m80-193. [DOI] [PubMed] [Google Scholar]
- Wang P. Y., Shopsis C., Schneider H. Fermentation of a pentose by yeasts. Biochem Biophys Res Commun. 1980 May 14;94(1):248–254. doi: 10.1016/s0006-291x(80)80213-0. [DOI] [PubMed] [Google Scholar]


