Abstract
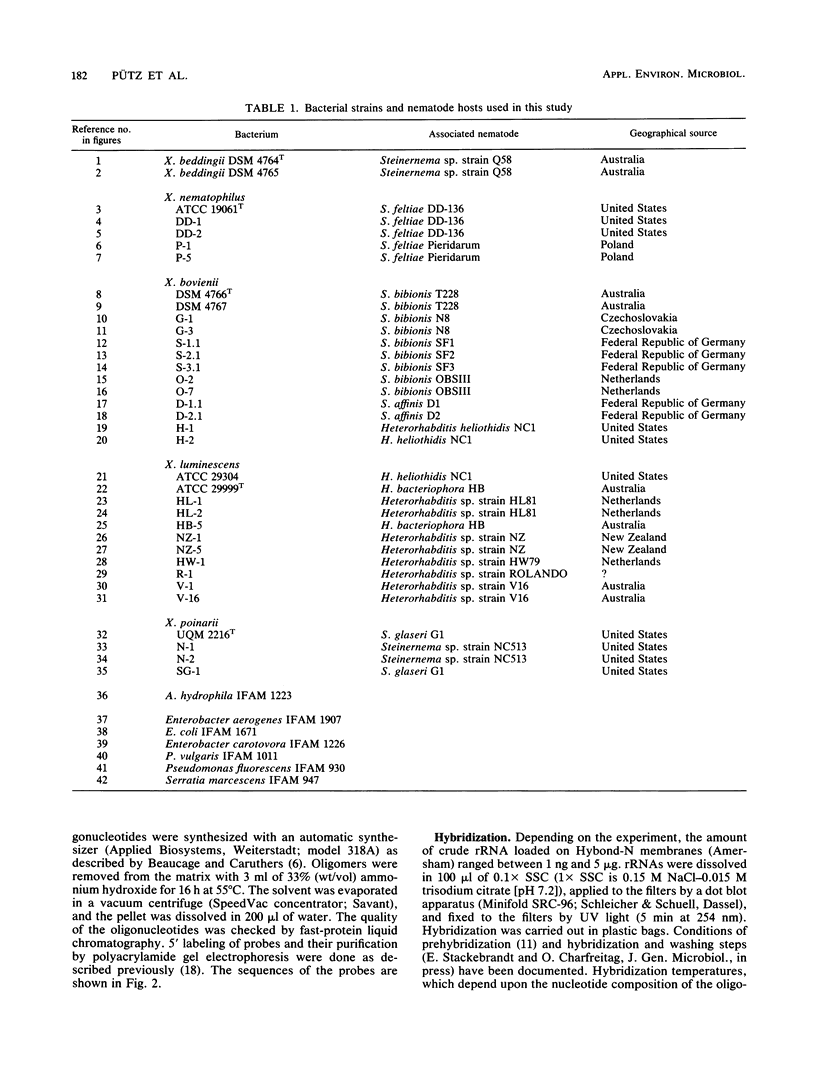
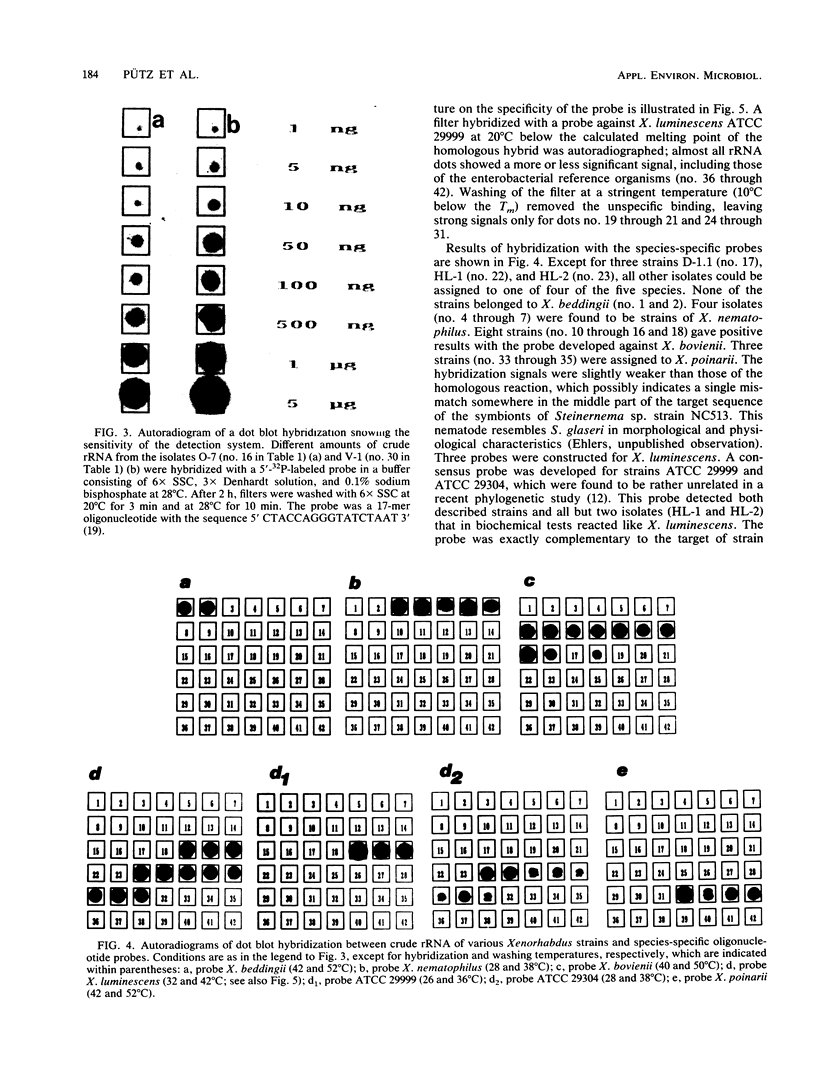
Synthetic deoxyoligonucleotide probes that hybridized against the region at positions 455 through 480 of 16S rRNA were developed for the identification of all five Xenorhabdus species. Sequence variation in the respective rRNA region between two strains of Xenorhabdus luminescens in addition allowed the construction of two strain-specific probes. Of 27 isolates determined to be Xenorhabdus strains by phenotypic characterization, 24 could be assigned to four of the five species. Two strains (HL-1 and HL-2) isolated from a Heterorhabditis sp. and a single strain (D-1.1) isolated from Steinernema affinis showed no hybridization signal with any of the five species-specific probes. With regard to the available species descriptions of nematodes, the results presented here confirm that, except for Steinernema affinis, the individual nematode hosts harbor only a single Xenorhabdus species.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhurst R. J. Antibiotic activity of Xenorhabdus spp., bacteria symbiotically associated with insect pathogenic nematodes of the families Heterorhabditidae and Steinernematidae. J Gen Microbiol. 1982 Dec;128(12):3061–3065. doi: 10.1099/00221287-128-12-3061. [DOI] [PubMed] [Google Scholar]
- Akhurst R. J., Boemare N. E. A numerical taxonomic study of the genus Xenorhabdus (Enterobacteriaceae) and proposed elevation of the subspecies of X. nematophilus to species. J Gen Microbiol. 1988 Jul;134(7):1835–1845. doi: 10.1099/00221287-134-7-1835. [DOI] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Lane D. J., Pace B., Olsen G. J., Stahl D. A., Sogin M. L., Pace N. R. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6955–6959. doi: 10.1073/pnas.82.20.6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poinar G. O., Jr, Thomas G. M. Significance of Achromobacter nematophilus Poinar and Thomas (Achromobacteraceae: Eubacteriales) in the development of the nematode, DD-136 (Neoaplectana sp. Steinernematidae). Parasitology. 1966 May;56(2):385–390. doi: 10.1017/s0031182000070980. [DOI] [PubMed] [Google Scholar]
- Szostak J. W., Stiles J. I., Tye B. K., Chiu P., Sherman F., Wu R. Hybridization with synthetic oligonucleotides. Methods Enzymol. 1979;68:419–428. doi: 10.1016/0076-6879(79)68031-x. [DOI] [PubMed] [Google Scholar]





